14 It’s Not Just the Tumor: CNS Paraneoplastic Syndromes and Cerebrovascular Complications of Cancers
It’s Not Just the Tumor: Syndromes and Complications
14.1 Introduction
A variety of neurologic complications and syndromes can be seen in patients with cancer. Other than direct tumor infiltration and/or metastatic spread, neurologic symptoms may arise in cancer patients due to a paraneoplastic cause or stroke and cerebrovascular complications, which in turn may be due to direct tumor-related causes or the side effects of tumor therapy. Stroke and cerebrovascular complications may be responsible for significant morbidity and mortality in patients with cancer and are the second most common pathology of the nervous system, seen in around 15% of cases in various autopsy series. 1 , 2
Paraneoplastic syndromes are the remote effects of the primary tumor and encompass various disorders unrelated to direct tumor invasion of the nervous system or resulting from the indirect effects of the cancer and its therapies, such as infections, coagulopathy, metabolic disturbance, or other side effects of treatment. 3 , 4 The term paraneoplastic was first introduced by Guichard and Vignon in a patient with uterine cancer presenting with multiple cranial and radicular neuropathies. 5 However, the first report of involvement of the peripheral nervous system in a patient with known malignancy dates back to late 19th century. 6 The pathogenesis of these syndromes is poorly understood; however, most studies have shown them to be due to immune response to the underlying malignancy. Knowledge of these entities in patients with cancer is crucial because their pathogenesis in this subgroup differs from that in the general population; hence their correct identification may be necessary for appropriate treatment to be initiated. The clinical manifestations of these neurologic complications may in fact precede the detection of primary tumor in a significant proportion of cases. Hence recognition of these syndromes is even more important because they provide a window for early detection of cancer and can impact patient management and survival.
Neuroimaging plays a crucial role in evaluation of cancer patients. In cases with extra central nervous system (CNS) malignancy presenting with neurologic symptoms, neuroimaging is used primarily to rule out the metastatic spread of disease. A computed tomographic (CT) scan is usually the first imaging test performed in these patients, and magnetic resonance imaging (MRI) is then performed for further evaluation and better characterization of the disease. Angiography or venography is performed in patients suspected to have stroke and cerebrovascular complications and can be noninvasive with the use of CT or magnetic resonance angiography (MRA) or venography techniques. Catheter angiography (digital subtraction angiography) is usually reserved for patients who need more detailed examination of the vasculature or patients needing endovascular therapeutic intervention.
This chapter describes various CNS paraneoplastic syndromes and cerebrovascular complications commonly seen in cancer patients with an emphasis on early diagnosis based on characteristic imaging features.
14.2 CNS Paraneoplastic Syndromes
14.2.1 Cerebellar Degeneration
Paraneoplastic cerebellar degeneration (PCD) is a well known entity associated commonly with small cell lung cancer, gynecological and breast cancers, as well as Hodgkin lymphoma. 7 This disorder is usually associated with the presence of anti-Yo, anti-Hu, or anti-Tr antibodies in the cerebrospinal fluid (CSF). The loss of Purkinje cells in the cerebellum is the pathognomonic finding on histology. Though the clinical manifestations (ataxia, diplopia, and dysarthria) are disabling, neuroimaging in the early stage of disease is usually unremarkable. Abnormal enlargement of the cerebellar hemisphere and corticomeningeal enhancement have been shown in some instances, 8 and a few case reports have shown fluid-attenuated inversion recovery (FLAIR) signal abnormalities in patients with fulminant cerebellar degeneration. Functional imaging techniques like positron-emission tomographic (PET) scan can help in earlier diagnosis of this entity. In the initial phase a fluorodeoxyglucose (FDG)-PET scan shows increased tracer uptake within the cerebellum, even in patients with a normal-appearing MRI scan. However, in the later stages, CT and MRI scans show diffuse cerebellar atrophy (most marked in midline), and PET scans demonstrate decreased tracer uptake. 9
14.2.2 Limbic Encephalitis
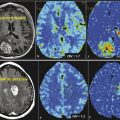
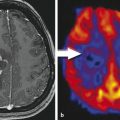
Paraneoplastic limbic encephalitis (PLE) is the inflammatory disorder confined to the limbic system due to autoimmune response to cancer cells. The malignancies commonly associated with this are small cell lung cancer, testicular germ cell neoplasms, thymoma, teratoma, and Hodgkin lymphoma. 10 Unlike most other CNS paraneoplastic disorders, the imaging studies are abnormal in the majority of patients with PLE. MRI usually shows bilateral but asymmetric increased T2 and FLAIR signal in the amygdala region and hippocampal formation of mesial temporal lobes (Fig. 14.1). Involvement of the unilateral temporal lobe can be seen as well and additionally the altered signal may also extend into other limbic structures like the insular cortex. 11 Contrast enhancement is rare and, if present, is usually minimal. Diffusion-weighted and high-resolution FLAIR sequences are shown to be better than conventional spin-echo sequences for earlier diagnosis of the condition. 12 Involvement of brain cortex prior to involvement of the temporal lobe in these patients has also been reported (Fig. 14.2). 13 A case series on PLE showed bilateral temporal lobe involvement in around 70% of cases, with extra temporal cortical signal abnormality in 37% and abnormal contrast enhancement in 21% of patients. 14 With time, the T2 hyperintense signal decreases and focal, temporal, or generalized cerebral atrophy ensues. An FDG-PET scan shows hypermetabolism in the affected areas. MRI signal abnormality in these patients does not vary with levels of oncoantibodies associated with limbic encephalitis. However, the tracer uptake on FDG-PET is shown to increase simultaneously with increase in the level of oncoantibodies. Hence the tracer uptake in these patients may also help to assess response to the treatment. 15


The temporal lobe may be affected by various other pathologies with overlapping clinical manifestations; imaging plays an important role in diagnosis and differentiation of these disorders. Infectious encephalitis (e.g., herpes), non-PLE mesial temporal sclerosis, and gliomatosis cerebri are other disorders that may have imaging similar to this. Differentiation of limbic encephalitis from herpes encephalitis is the primary consideration. PLE tends to have multifocal brain involvement, lesser local mass effect, and a lesser degree of contrast enhancement on MRI scans. The presence of associated antibodies and a history of cancer (if already diagnosed) may further add to the diagnosis of PLE. 11
14.2.3 Brainstem Encephalitis
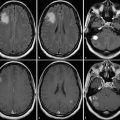
Paraneoplastic brainstem encephalitis is often seen along with the presence of other paraneoplastic syndromes like limbic encephalitis, cerebellar degeneration, or multifocal encephalomyelitis. 16 It is seen in patients with small-cell carcinoma of the lung, testicular tumors, breast cancer, hypernephroma, or prostate cancer and is commonly associated with the presence of anti-Hu, anti-NMDAR, or anti-Ma2 antibodies in serum or CSF. 17 , 18 MRI may show T2/FLAIR hyperintensity in the midbrain tectum, periaqueductal gray matter, substantia nigra, pons, medulla, and superior/middle cerebellar peduncles. Contrast enhancement is usually not seen, but nodular enhancement along the structures just described has also been reported. 19 , 20 Though uncommon, involvement of the cortex or the juxtacortical white matter (Fig. 14.3) along with involvement of brainstem may be seen in patients with onconeural antibodies. The radiological differential diagnosis of paraneoplastic brainstem encephalitis includes infectious encephalitis, vasculitis, demyelination, or low-grade glial neoplasm.

14.2.4 Striatal Encephalitis or Chorea
Paraneoplastic striatal encephalitis is most often seen in patients with small cell lung cancer and thymoma and is commonly associated with presence of anti-CV2/CRMP-5 antibodies. 21 MRI in these patients is often abnormal and may show T2/FLAIR signal abnormality in bilateral caudate nuclei and putamen. The signal abnormality may also extend into the adjacent white matter. There is usually no contrast enhancement or restricted diffusion (increased signal) on diffusion-weighted imaging (DWI). 17 Basal ganglia signal changes are seen to resolve with clinical improvement of the patient condition. With this imaging appearance, the radiological differential diagnosis of paraneoplastic striatal encephalitis includes viral encephalitis, sporadic Creutzfeldt-Jakob disease (CJD), acute disseminated encephalomyelitis (ADEM), anoxic brain injury, and various metabolic conditions. Absence of restricted diffusion and extension of signal abnormality into the adjacent white matter help to distinguish paraneoplastic striatal encephalitis from prion disease. On MRI scans the presence of signal abnormalities associated with other paraneoplastic CNS disorders may also help to distinguish paraneoplastic striatal encephalitis from other radiological differentials.
14.2.5 Paraneoplastic Myelitis
Paraneoplastic myelitis is a severely disabling disorder of the spinal cord and is associated with various cancers (lung, breast, kidney, and ovary) in the literature. 22 , 23 Symmetric, longitudinally extensive tract or gray matter-specific T2/FLAIR hyperintensity, which may enhance on contrast administration, has been described in these patients on spine MRI. 20
14.2.6 Hypertrophic Polyneuropathy
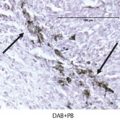
Hypertrophic polyneuropathy is a rare paraneoplastic neurologic disorder that is characterized by thickening and necrosis of the involved nerve roots. It has been reported with lung tumor, carcinoid, melanoma, lymphoproliferative malignancies, and malignant thymoma. 24 , 25 , 26 , 27 Characteristically, inflammatory and malignant cells are absent within the involved nerves, distinguishing it from inflammatory demyelinating polyneuropathy or a metastatic disease, which are common causes of neuropathy. Although there is no classical onconeural antibody associated with hypertrophic polyneuropathy, various nonspecific antibodies may be seen in patients with paraneoplastic neuropathy. There may be involvement of single or multiple nerve roots, and there is varying involvement of sensory and motor nerve fibers. Selective involvement of multiple cauda equina nerve roots has been reported. 26 , 27 , 28 On MRI scan, hypertrophic polyneuropathy is characterized by thickening of the involved nerve roots, which enhance on contrast administration (Fig. 14.4). Hypertrophy and enhancement of these nerve roots have been reported to resolve following resection of the primary tumor. 29

14.3 Tumor-Related Stroke and Cerebrovascular Complications
14.3.1 Cerebral Venous Thrombosis
Cerebral venous thrombosis (CVT) may occur due to a hypercoagulable state in cancer patients or it may be because of direct invasion of the dural venous sinuses by primary brain neoplasm or metastatic deposits in meninges/calvarium. 29 Patients with CVT may present with headache, convulsions, focal neurologic deficits, or coma. The presence of thrombosis of vessels of extra-CNS organ systems may be a useful clue to diagnosis.
Noncontrast CT (NCCT) is usually the first imaging investigation in these patients and may show a hyperdense thrombus within the dural venous sinus or the cortical vein. Further, there may be infarction (hemorrhagic or nonhemorrhagic) within the brain parenchyma that does not conform to any arterial territory. On contrast administration, there may be enhancement of the margins of the dural venous sinus (known as the empty-delta sign) due to collateral veins within the walls of the dural venous sinus and prominent tentorial enhancement due to retrograde venous congestion. 30 However, conventional CT findings may be insignificant in a third of these patients. With advances in CT technology, CT venography can be quickly obtained in these patients and with faster scans and isotropic acquisition; three-dimensional reformats can be obtained from the data. CT venography is not limited by flow artifacts as in MRI, is faster to obtain, and may be obtained in the setting of the initial CT study if the clinical suspicion is high. CT venography has been shown to be equally as good as MR venography in these patients in various studies. 31 , 32 , 33
MRI is better for detection of parenchymal changes in these patients. On conventional fast spin-echo sequences, thrombosed venous sinuses are seen as loss of normal flow voids. In the very early stage (< 5 days), the thrombosed sinus appears isointense on T1-weighted MRI and hypointense on T2-weighted MRI and hence may be missed on conventional MRI. However, MR venography overcomes this and shows nonvisualization of thrombosed sinus (Fig. 14.5). MRI is also better for delineation of parenchymal changes (edema, hemorrhagic, or nonhemorrhagic infarct) of the CVT. 34 DWI is a relatively new tool in imaging of patients with CVT. On DWI, the brain parenchyma shows heterogeneous signal with increased or normal apparent diffusion coefficient (ADC). These signal changes signify the presence of a combination of vasogenic and cytotoxic edema. The combination of these MRI sequences is usually helpful in diagnosis of cerebral venous sinus thrombosis. But in equivocal cases or in patients with isolated cortical venous thrombosis, conventional catheter angiography may be required. The direct sign of cerebral sinus thrombosis is nonvisualization of the venous sinus. The other indirect signs, which may point to the occlusion of the cerebral venous system, are dilatation of collateral veins with a corkscrew appearance, delayed venous emptying, and dilation of collateral circulation. 35 Another possible mechanism of cerebral sinus thrombosis in patients with tumor is by direct invasion or compression of the venous structures by parenchymal, dural, or calvarial metastases. CT and MRI both may show the enhancing mass responsible for venous thrombosis. However, these imaging modalities may not be able to distinguish bland and tumor thrombus. There may be enhancement of tumor thrombus, but the same may also be seen in the subacute stage of bland thrombus.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree