PLEURAL PHYSIOLOGY AND PLEURAL EFFUSIONS
PLEURAL PHYSIOLOGY AND PLEURAL EFFUSIONS (Box 15.1)
The outward pull of the chest wall and the inward recoil of the lung tend to separate the parietal and visceral pleura. These membranes are permeable to both gases and liquid, and are kept in apposition only because of mechanisms that keep the pleural space essentially free of gas and liquid. Gas is removed from the pleural space by systemic venous blood because the total gas pressure in venous blood is about 70 cmH2O subatmospheric, and this provides a steep absorption gradient. The mechanisms governing the formation and absorption of pleural fluid are more complex.
Box 15.1
• Up to 10 mL of fluid may be found in the pleural space of normal subjects
• Pleural effusions develop when the rate of formation of fluid and its resorption are mismatched
• Transudative effusions are usually due to systemic disease causing increased formation of pleural fluid
• Exudative effusions are typically due to pleural inflammation or malignancy
• Biochemical markers may help in diagnosis of a pleural effusion but none is entirely specific
The pleural space is lubricated by a small amount of pleural fluid. This liquid coupling provides instantaneous transmission of perpendicular forces between pleural surfaces and allows the pleural membranes to slide in response to shear forces. 1 The volume of pleural fluid in the human pleural space, determined by urea dilution, is 0.26 mL/kg. 2 This means that normal individuals may have 8–10 mL of fluid in each pleural space. Using lateral decubitus chest radiographs to detect pleural fluid, a technique that has a threshold sensitivity of about 5 mL, 3 Hessen4 found a 10% prevalence of definite or probable pleural fluid in healthy adults. In humans, it is estimated that 10–20 mL of pleural fluid is formed each day.5.6. and 7. Normal pleural fluid has a protein concentration of 1–2 g/dL, a cell count of 1500–4500/mL (60–70% monocytes) and less than half the serum concentration of large protein macromolecules such as lactate dehydrogenase (LDH). 8
Pleural fluid originates as extracellular interstitial fluid in parietal pleural tissue, and leaks from there into the pleural space through nontight mesothelial junctions. Physiologically, the pleural space is best considered as part of the parietal pleural extracellular space.6. and 9. Under physiologic conditions, the rate of fluid formation by the visceral pleura is low because the bronchial arteries which supply the pleura are relatively deep to the surface, and the pleural capillaries, draining into pulmonary veins, have low pressure. 10 Removal of pleural fluid is primarily through parietal pleural lymphatics.
Lymphatic drainage of the pleural space allows removal of proteins, particulates, and cells in addition to water and crystalloids. Protein is also removed by active transport by mesothelial cells. 10 Protein removal is particularly important in pathologic states, but even under normal conditions some protein leaks into the pleural space. Were this not removed, the subsequent rise in oncotic pressure in the pleural fluid would lead to progressive pleural fluid accumulation.
Pleural effusions develop when the rate of entry and exit of pleural fluid is mismatched, because of increased microvascular hydrostatic pressure, reduced oncotic pressures, impaired lymphatic drainage, and increased mesothelial or vascular permeability.6. and 10. Less common mechanisms include reduced pressure in the pleural space (seen with major atelectasis), and transdiaphragmatic passage of fluid from the peritoneum. A variety of liquids may accumulate in the pleural space: transudate, exudate, blood, chyle, and occasionally bile, urine, cerebrospinal fluid, peritoneal dialysate, and intravenous infusions. Because the character of pleural fluid is often unknown, liquid in the pleural space is usually called pleural effusion. Alternative and more specific terms such as hemothorax, pyothorax, and chylothorax may be used as appropriate.
Distinction between transudative and exudative pleural fluid is pivotal for diagnosis and management of effusions. The standard criteria for distinguishing transudates from exudates were defined by Light et al.5. and 11. (Box 15.2). In a multicenter metaanalysis, the overall diagnostic accuracy of the three-test Light criteria was 92%. 12 However, they appear to be more accurate for diagnosis of exudates than for transudates. For example, in a study by Romero et al., 13 98% of exudates and 77% of transudates were correctly classified by these criteria. Most misclassified transudates have criteria close to the cutoff values. A number of studies have shown that transudative effusions may acquire the biochemical characteristics of an exudate if they are longstanding6 or if the patient has received diuretics.14. and 15.
Box 15.2
Transudates develop because of a change in the physical factors that affect the rate of pleural fluid formation and resorption: microvascular pressure and plasma oncotic pressure. Because of the generalized nature of these changes, transudates are commonly bilateral. The important causes of transudative pleural effusions are listed in Box 15.3. Management of transudative pleural effusions is generally based on treatment of the underlying systemic condition.
Box 15.3
• Raised microvascular pressure
– Heart failure
– Constrictive pericarditis
– Fluid overload
• Reduced plasma oncotic pressure
– Hepatic cirrhosis
– Nephrotic syndrome
– Hypoalbuminemia (other causes)
• Reduced pleural surface pressure
• Major atelectasis (e.g. total lung collapse)
An exudative effusion indicates that the pleural surface is pathologically altered, with an accompanying increase in permeability or a decrease in lymph flow. The typical and less common causes of exudative effusions16.17.18.19.20.21.22.23. and 24. are shown in Box 15.4.
Box 15.4
Infectious
• Bacteria (including Mycobacteria)
• Viruses, Chlamydia
• Fungi
• Protozoa (including Pneumocystis jirovecii), metazoa, worm infestation
• Adjacent subphrenic abscess, hepatic abscess (including amebic), vertebral osteomyelitis
Neoplastic
• Bronchial carcinoma
• Mesothelioma
• Metastasis
• Lymphoma
• Localized fibrous tumor of pleura
• Castleman disease, macroglobulinemia
• Chest wall neoplasm
Cardiovascular
• Postcardiac injury syndrome
• Superior vena cava obstruction
• Pulmonary thromboembolism and infarction
Hepatic
• Cirrhosis
• Hepatitis
Pancreatic
• Pancreatitis (acute, chronic)
Renal
• Uremic pleurisy
• Acute nephritic syndrome
• Renal tract obstruction
• Renal infection
Splenic
• Abscess
• Infarction
• Hematoma
Ascitic
• Cirrhosis
• Meigs syndrome
• Ascites (benign, malignant)
• Peritoneal dialysis
Traumatic
• Open or closed chest trauma, including diaphragmatic hernia
• Esophageal rupture
• Iatrogenic, including thoracic and abdominal surgery, esophageal sclerotherapy, radiation
Inhalational
• Asbestos
Inflammatory
• Rheumatoid disease
• Systemic lupus erythematosus
• Scleroderma
• Wegener granulomatosis
• Idiopathic lobular panniculitis (Weber–Christian disease)
Drug-induced
• Methotrexate and other drugs
Miscellaneous
• Sarcoidosis
• Myxedema
• Yellow nail syndrome
• Familial Mediterranean fever
• Atelectasis
• Gorham syndrome
• Ovarian hyperstimulation syndrome
• Peripartum pleural effusion
In practice, more than 90% of effusions (whether symptomatic or asymptomatic) result from heart failure, cirrhosis, ascites, pleuropulmonary infection, malignancy, or pulmonary embolism.5.25. and 26. Very large effusions are seen especially in malignant disease as a result of metastases, notably from the lung and breast, but large effusions may also occur in heart failure, cirrhosis, tuberculosis, empyema, and hemothorax. 27 Bilateral effusions, if not transudates, may be due to metastatic disease, lymphoma, pulmonary embolism, rheumatoid arthritis, and systemic lupus erythematosus.
The differential diagnosis of pleural effusion depends on patient history, physical examination, and a hierarchy of investigations that, depending on possible diagnoses, include blood and urine tests, thoracentesis with an examination of the pleural fluid, and pleural biopsy. Exudates should be examined for glucose concentration, microorganisms, malignant cells, and in appropriate circumstances amylase and triglycerides. 9 If the effusion is thought to be immunologic in origin, measurement of complement levels and autoantibodies may be helpful. Measurements of adenosine deaminase, lysozyme, and γ-interferon may assist the diagnosis of tuberculous pleural effusion. 5 If initial evaluation is nondiagnostic, other investigations that may be used include thoracoscopy, bronchoscopy, and open pleural biopsy. Image-guided pleural biopsy may have a very high diagnostic yield. 28 Imaging procedures play an early and important part in this workup and may disclose abnormalities both inside and outside the chest that can establish or suggest a probable cause for the effusion. In addition, imaging techniques are used to direct biopsy or interventional therapy.
A subset of exudative pleural effusions are eosinophilic (i.e. when eosinophils make up more than 10% of the white cell count in the pleural fluid). In one review of 343 eosinophilic effusions the majority were either idiopathic (35%) or associated with pneumothorax or hemopneumothorax (28%). 29 Other causes included parapneumonic effusion (5%), tuberculosis (4%), malignancy (8%), pulmonary infarction (4%), benign asbestos-related effusion (4%), and rheumatologic disease (4%). Eosinophilic pleural collections have been also described in infections with the round worm Toxocara canis30 and following trauma. 31 In general, eosinophilic effusions are self-limiting. 6 However, Kuhn et al. 32 have emphasized the importance of disease prevalence in making judgments concerning the significance of an eosinophilic effusion. In their study, although only four of 84 effusions in patients with malignancy were eosinophilic, 48% of those with eosinophilic effusion were malignant. 32
Several large series indicate that 10–20% of pleural effusions examined by thoracentesis are of indeterminate cause despite extensive investigation.26.33.34. and 35. In a prospective review of patients with pleural effusions, a diagnosis was not initially established in 53 out of 394 (13%) cases. 36 In 32 out of 40 (80%) of these patients, no cause was ever determined despite a mean follow-up of 62 months. In general, there was complete resolution of pleural fluid in all patients. 36 Relapse of pleural effusion was documented in five out of 40 (12%) patients, of whom one was diagnosed with mesothelioma.
Imaging of pleural effusion (Box 15.5)
Conventional radiography, computed tomography (CT), and ultrasound are specific and sensitive ways of demonstrating pleural fluid. The appearance of an effusion depends on the patient’s position at the time of the examination and the mobility of the effusion, which may be free or constrained to a variable extent.
Box 15.5
• Chest radiography is the primary method for detection of pleural fluid
• Decubitus views help confirm mobility of fluid
• Ultrasonography will detect small volumes of pleural fluid, and may help to differentiate between a transudate and an exudate
• CT will detect small pleural effusions and evaluate morphology of the pleura and underlying lung parenchyma
• MRI is rarely of value, except in mesothelioma.
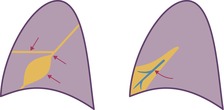
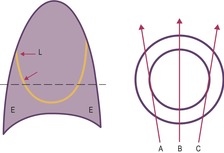
With a moderate-sized pleural effusion, fluid collects around and under the lung and takes on a characteristic configuration, determined by the interplay of the hydrostatic pressure in the effusion, the pleural liquid pressure, and the pleural surface pressure in the various zones from apex to base. In the erect subject, fluid collects mainly in the lower zone. Here the hydrostatic pressure is positive and the lung is compressed, so that it floats away from the chest wall and diaphragm.1. and 37. At the lung apices, intrapleural pressure remains negative, and any fluid is minimal. In the mid-thorax, pleural pressure becomes less negative, and elastic recoil causes the lung to fall away from the chest wall, allowing fluid to intervene between the visceral and parietal pleura. As lung volume decreases in the more caudal lung, the thickness of pleural fluid increases progressively. Because of these interactions between pulmonary elastic recoil and increased intrapleural pressure, the lung eventually sits in the pleural fluid a bit like an egg in an egg cup, and the upper surface of the effusion acquires a meniscus-like shape (Fig. 15.1).
 |
| Fig. 15.1 (From Wilson AG. Br J Hosp Med 1987;37:526–534.) |
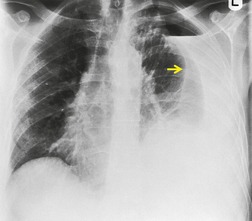
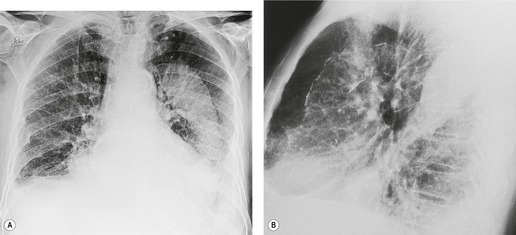
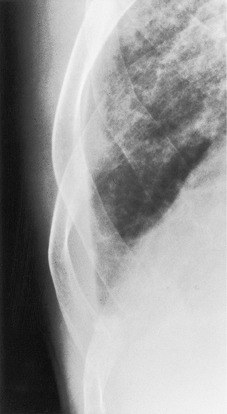
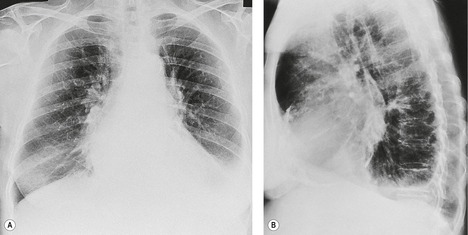
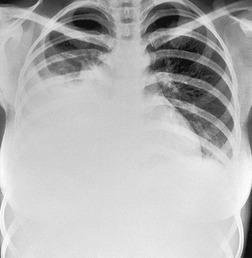
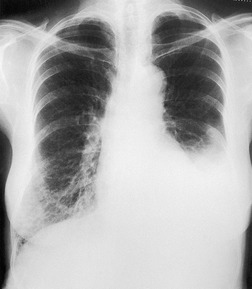
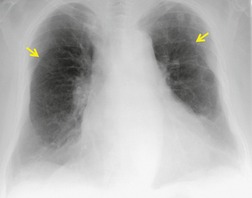
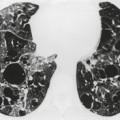
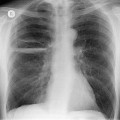
On erect chest radiographs, the classic appearance of a moderate pleural effusion is a homogeneous lower zone opacity with a curvilinear upper border, quite sharply marginated and concave to the lung (Fig. 15.2). A consideration of Figure 15.1 explains how this type of opacity is produced. The transaxial view shows that the X-ray beam is more attenuated laterally (A) than centrally (C) because the marginal beam passes through a greater thickness of fluid. A pleural effusion is therefore dense laterally, and because it presents a tangential lung–fluid interface (B), it has a sharp inner margin with the classic meniscus shape produced by the retracted lung surface. More centrally, the X-ray beam is less attenuated by the relatively thin anterior and posterior fluid collections, and the effusion produces only a general haziness, the upper edge of which cannot be seen because it has wedge-shaped geometry (Fig. 15.2). The meniscus is usually higher laterally than medially because lung attachments at the hilum and inferior pulmonary ligament alter the distribution of forces (Fig. 15.2).
 |
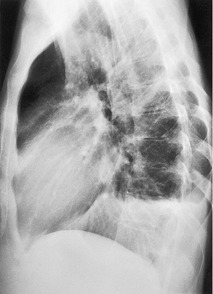
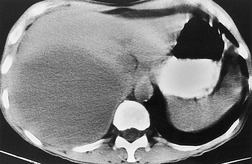
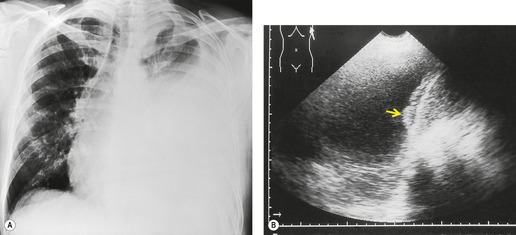
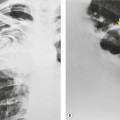
| Fig. 15.2 Tuberculous pleural effusion. Right-sided opacity has classic features of free pleural effusion in erect patient. Opacity is homogeneous, occupies inferior part of the chest, and has a concave upper margin that extends higher laterally than medially. The lung–fluid interface is clearly defined where the meniscus is seen in profile along the lateral chest wall, but is poorly defined elsewhere, because the meniscus is seen en face (see Fig 15.1). Note the left paraspinal widening, which was found to represent a tuberculous paravertebral abscess. |
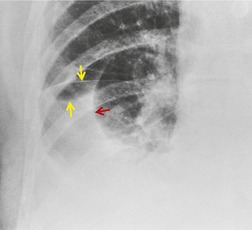
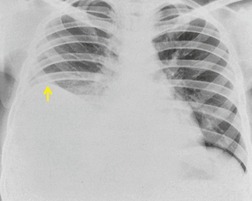
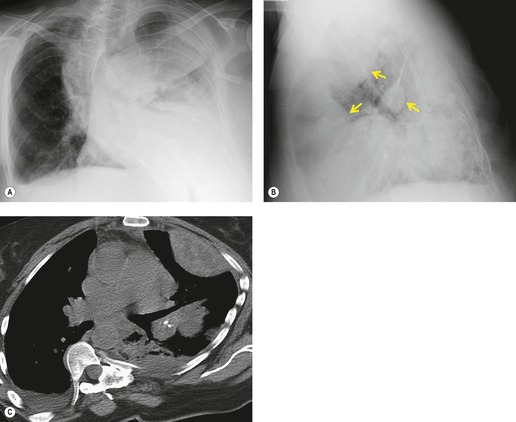
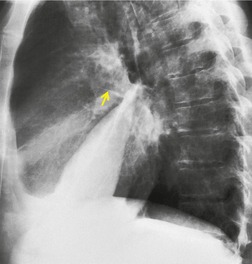
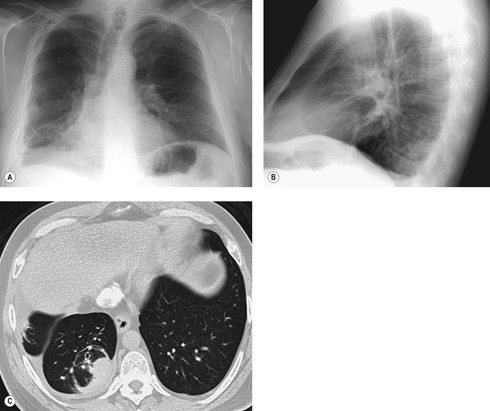
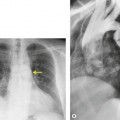
If there is free pleural fluid within fissures, the radiographic appearance depends on the shape and orientation of the fissure, the location of the fluid within it, and the direction of the X-ray beam. 38 Fluid in the major fissure may cause a curvilinear interface on a frontal chest radiograph (Fig. 15.3). Such an opacity may be faint; typically, it is relatively lucent medially and more dense laterally and superiorly. 38 The medial curvilinear interface marks the limit of fluid intrusion into the fissure, which may or may not be at the point of fissural fusion. 39 A higher and more peripheral curvilinear interface may be produced by fluid invagination into the upper end of the major fissure. 40 Such tonguelike intrusions of fluid into the margins of fissures are common and may be seen early in the development of a pleural effusion. 1 Intrusion of fluid into the lateral aspect of the minor fissure gives rise to the thorn sign on a frontal radiograph. 41 A more complex shadow also attributed to fissural intrusion is the middle lobe step (Fig. 15.4). This is seen on the lateral view, and consists of a steplike accumulation of fluid anteriorly below the minor fissure, thought to result from overlapping fluid intrusion into incomplete major and minor fissures.38. and 40. Moderate or large right or left pleural effusions may displace the azygoesophageal interface, producing a contralateral retrocardiac masslike lesion (Fig. 15.5). 42 Fluid within the azygoesophageal recess may also simulate subcarinal adenopathy. 42
The radiographic appearance of pleural fluid may be modified when there is associated lung atelectasis. 43 The increased elastic recoil of the atelectatic lung results in an atypical distribution of fluid. Another unusual appearance with moderate-sized effusions is circumferential encapsulation of a lobe, particularly the lower lobe, simulating consolidation.38.39. and 40. Sometimes fluid collects preferentially against the mediastinum, particularly on the left, causing a triangular retrocardiac density simulating lower lobe collapse, from which it can be differentiated by the lack of depression of the lower lobe bronchus. This distribution of fluid has been attributed to the modifying influence of the pulmonary ligament.44. and 45.
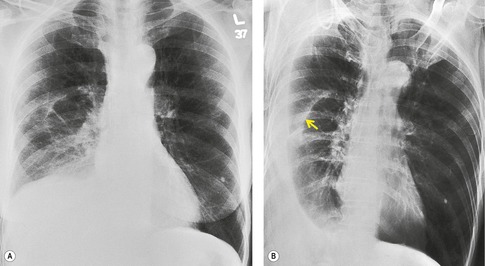
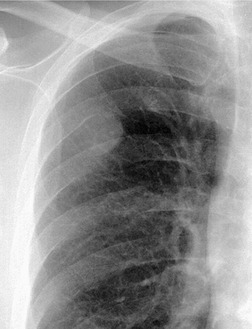
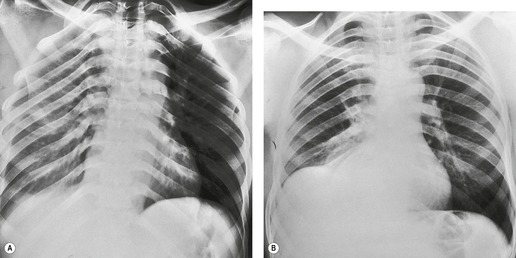
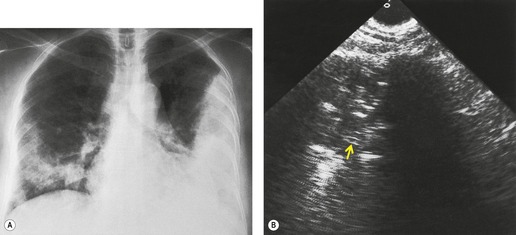
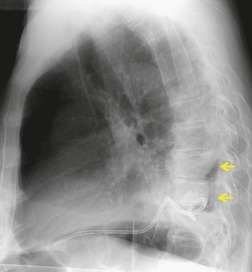
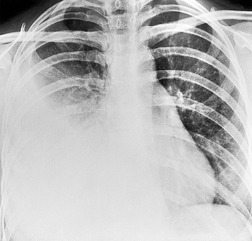
Early in their development pleural effusions are small and collect in the subpulmonic space, between the lower lobes and the diaphragm (Fig. 15.6).37. and 46. After a variable amount of fluid has accumulated, it spills into the posterior and then into the lateral costophrenic angles and may cause subtle changes in the contour of the inferior aspect of the lower lobe. Because the posterior costophrenic sulcus is deeper than the lateral sulcus, the lateral chest radiograph is more sensitive than the frontal radiograph for detection of pleural effusion. The only study that has assessed the amount of pleural fluid needed to blunt the costophrenic angles on posteroanterior radiographs is a postmortem study in erect cadavers in which it was found that on average the required volume was 175 mL (range 25–525 mL). 47
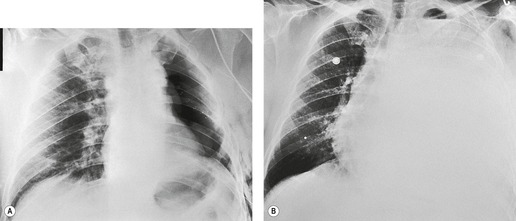
When effusions are too small to be detected on conventional posteroanterior and lateral chest radiographs, they may be demonstrated with special radiographic views or by ultrasound or CT. The most widely used special radiographic view is the lateral decubitus (Fig. 15.4), 48 which can detect 3–10 mL of fluid.3. and 48. Kocijancic et al., 49 in a study of 36 patients with small pleural effusions, found that expiratory decubitus radiographs were more sensitive than inspiratory decubitus images for detection of fluid. The sensitivity of ultrasound, performed with careful technique in a semidecubitus position, is similar to that of decubitus radiographs. 50
Subpulmonic effusion
Occasionally, for reasons that are obscure, large quantities of pleural fluid accumulate in a subpulmonic location rather than escaping into the general pleural cavity. Subpulmonic effusions are often transudates and associated with renal failure, liver cirrhosis, congestive heart failure, and nephrotic syndrome. 48 There are, however, many exceptions. Subpulmonic effusions may be unilateral or bilateral; when unilateral they are more commonly right sided, and when bilateral they can easily be missed on the chest radiograph. 48
Subpulmonic effusions can be difficult to detect because the upper edge of the fluid mimics the contour of the diaphragm on the chest radiograph (Fig. 15.6), so that the principal sign is an apparent elevation of the hemidiaphragm, 48 with the minor fissure appearing closer to the apparent diaphragm than usual. The ‘elevated hemidiaphragm’ may have one or more of the following features, which should suggest the correct diagnosis (Box 15.6):
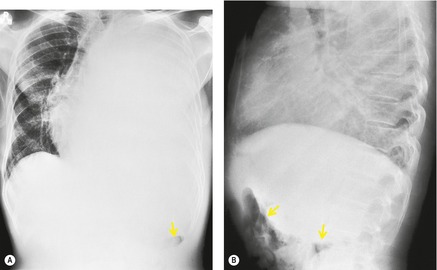
• The peak of the ‘hemidiaphragm’ is more lateral than usual (Figs 15.6, 15.7, see also Fig. 15.24B), and the contour on either side of the peak is straight. The medial slope tends to be gradual whereas the lateral one is steep.51.52. and 53. These appearances, particularly the lateral peaking, are accentuated on expiration, 53 a phenomenon that has been attributed to the presence of the inferior pulmonary ligament.
• The costophrenic angle is usually ill defined, blunted, or shallow, 48 but exceptionally both the lateral and the posterior angles can be clear.
• On the left side, there is increased distance between the lung and the gastric air bubble. Unfortunately, this distance is variable in healthy patients. Comparison with previous radiographs may help assessment. Some authors suggest that a distance of more than 2 cm is suggestive of subpulmonic effusion. 55
• The frontal radiograph occasionally shows a diaphragmatic spur associated with fluid entering the inferior accessory fissure48 or a more substantial triangular retrocardiac shadow. This latter opacity, which effaces the medial ‘hemidiaphragmatic’ contour and the left paravertebral interface, results from paramediastinal extension of the subpulmonic fluid. 56
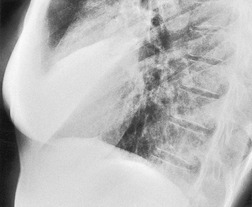
• On the lateral radiograph, the posterior aspect of the ‘hemidiaphragm’, beneath the lower lobe, tends to be flat, due to filling of the posterior costophrenic sulcus (Fig. 15.8). At the major fissure the silhouette usually slopes steeply downward, and there may be a tail of fluid passing up into the fissure itself.51. and 57.
Box 15.6
• Apparent elevation of hemidiaphragm
• Lateral peaking of ‘raised hemidiaphragm’
• Nonvisibility of pulmonary vessels behind ‘hemidiaphragm’
• Blunting of lateral or posterior costophrenic sulcus
• On the left side, increased distance from lung to gastric bubble
Sulpulmonic pleural fluid is usually not loculated, and the diagnosis may therefore be confirmed by taking a decubitus radiograph (Fig. 15.9B). 4 Alternatively, its presence may be confirmed by ultrasound or CT.
Large pleural effusion
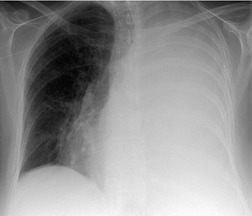
Large pleural effusions obscure the border of the heart and displace the mediastinum, airways, and diaphragm. Large pleural collections can have significant hemodynamic effects and, depending on the position of the patient, may influence gas exchange.58.59.60. and 61. Large pleural effusions should lead to contralateral displacement of the mediastinum (Fig. 15.5), and may cause a mediastinally based retrocardiac density as a result of herniation of the fluid-filled azygoesophageal recess. 62 A central mediastinum in the presence of a large pleural effusion suggests either that the mediastinum is fixed (most commonly the result of malignant infiltration by carcinoma or mesothelioma) or that there is substantial associated obstructive lung collapse, often because of an obstructing lung cancer. 48 Identification of obstructive lung collapse in association with effusion is important, because it suggests that the lung will likely not expand in response to thoracentesis, and a negative pressure pneumothorax may develop. In contrast, relaxation collapse of the lung, which normally accompanies a large pleural effusion, is not usually so great that it makes up for the volume of the effusion. On lateral views posterior pleural collections cause anterior displacement of central airways, a helpful differentiating feature from collapse or consolidation. 63
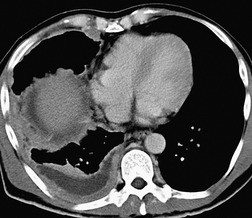
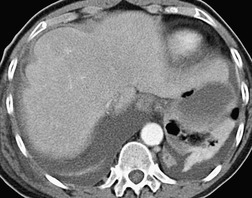
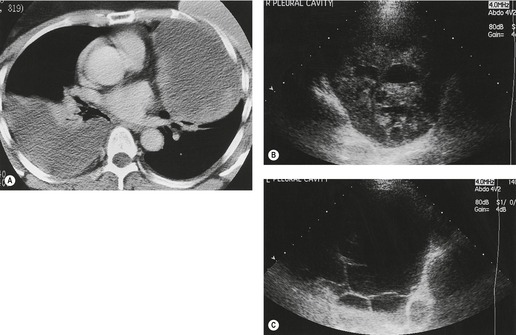
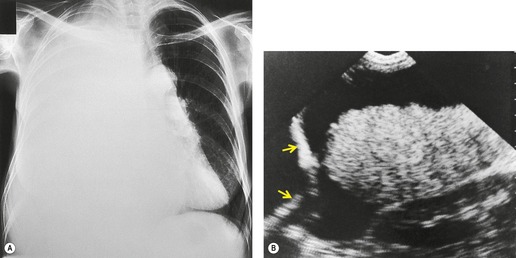
Inversion of the hemidiaphragm is an occasional but well-recognized finding.57. and 64. It is seen with large or moderate effusions, more commonly on the left than on the right, 48 a difference ascribed to the lack of liver support on the left. 65 Diaphragmatic inversion is easier to recognize on the left, where displacement of gastric and colonic gas indicates the shape and position of the left hemidiaphragm (Fig. 15.10). On the right side it is most easily diagnosed by ultrasonography (Figs 15.11 and 15.12).65.66. and 67. On CT examination, an inverted right hemidiaphragm must be distinguished from a cystic liver lesion (Fig. 15.13).68.69. and 70. Other lesions that may invert the hemidiaphragm include pneumothorax, lobar emphysema, diaphragmatic neoplasm, pericardial cyst, and myocardial aneurysm. 71 Diaphragm inversion generally produces few symptoms. However, it may stimulate an upper abdominal mass,72. and 73. or lead to paradoxic diaphragmatic movement67 that could cause dyspnea because of pendulum breathing (expired air from the ipsilateral lung being inspired contralaterally). If the inversion disappears following thoracentesis, the size of the pleural effusion on chest radiograph may appear unchanged.
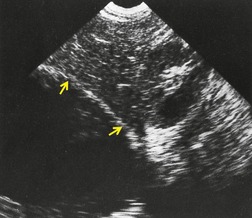 |
| Fig. 15.11 (Courtesy of Dr. A E A Joseph, London, UK.) |
 |
| Fig. 15.12 (Courtesy of Dr. A E A Joseph, London, UK.) |
Loculated pleural effusion
Pleural fluid may loculate within the fissures, or between parietal and visceral pleura when the pleural layers are partly fused. Loculations occur most typically with exudative pleural effusions, particularly parapneumonic effusions or hemothorax. However, transudative effusions may also loculate, particularly if there has been previous pleural inflammation.
Loculations against the chest wall are the most frequent. They take on a variety of configurations, most commonly a dome-shaped projection into the lung (Fig. 15.14). The radiographic appearance depends on the radiographic projection. If the loculation is seen en face or obliquely, it produces a rounded opacity, often with sharp inferomedial and ill-defined superolateral margins (Fig. 15.15). When viewed tangentially, a loculation produces a sharply demarcated convex interface with the lung. These lesions tend to have greater length than thickness, and because of the weight of contained fluid the greatest thickness may be towards the inferior part of the loculation. The margins of the loculated collection make an obtuse angle with the chest wall, elevating a ‘tail’ of pleura (Fig. 15.14C). Loculated pleural effusions share many radiographic features with chest wall or pleural masses, 74 and they are often indistinguishable. Extrapleural chest wall lesions, however, tend to have thickness more equal to their length, to have their greatest depth opposite the center of attachment, and may show rib destruction (Fig. 15.16). 74 Loculated pleural fluid is typically accompanied by evidence of fluid elsewhere in the pleural cavity.
Pleural fluid loculation within fissures occurs most commonly in heart failure.75. and 76. Because these fissural collections tend to come and go, they have been called vanishing or phantom tumors, or pseudotumors. 75 Such loculations are seen more commonly on the right and in the minor rather than the major fissure despite the greater area of the latter (Fig. 15.17). 75 A fluid collection in the minor fissure on the frontal view may simulate a pulmonary mass because of its sharply demarcated rounded or oval outline. The lateral view is helpful in diagnosis because of the characteristic lenticular shape of intrafissural fluid, often with a pathognomonic tail extending along the fissure for a short distance. Usually the diagnosis is easily made if the distinctive shape and position of the lesion are considered, particularly in the presence of heart failure.
Loculated collections in the major fissure have the expected distinctive lenticular configuration on the lateral view (Figs 15.18 and 15.19). On the frontal projection, the opacity may be either hazy and veil-like or more discrete and masslike (Fig. 15.19). Loculations in the caudad part of the major fissure (Fig. 15.20) may resemble middle lobe collapse or consolidation (Fig. 15.21). In this situation the following points favor loculated fluid rather than collapse (Fig. 15.22): (1) identification of a separate minor fissure (Fig. 15.18); (2) one or more convex margins in lateral projection (with collapse, usually at least one border is concave or straight); (3) right border of the heart not effaced on frontal view; and (4) both ends of the opacity tapering in lateral view (with collapse the anterior end is usually broad). 57 Two findings that favor collapse are (1) contact between the anterior end of the shadow and the chest wall and (2) an air bronchogram (Fig. 15.22). 57 Loculated pleural fluid along the diaphragm and mediastinum, though less common, may simulate a diaphragmatic or mediastinal mass. The appearances are similar to loculations along the chest wall.
Thickening of the subpleural space due to interstitial lung edema may sometimes cause soft tissue thickening of the lower lateral chest wall mimicking a pleural effusion (Fig. 15.23). This entity was previously called a lamellar pleural effusion, but may be distinguished from pleural effusion by its straight margin parallel to the chest wall, and by the lack of blunting of the costophrenic angle.
Pleural effusion in the supine patient
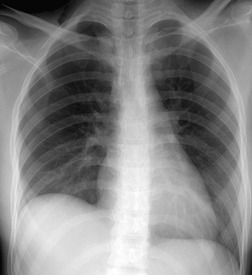
When a patient is supine, free pleural fluid layers posteriorly, and although a meniscus effect occurs at the lung–fluid interface, it is not appreciated on a frontal projection because it is oriented at right angles to the X-ray beam. The supine chest radiograph is not particularly sensitive or specific in the diagnosis of pleural effusion, 77 although in one study 90% of effusions between 200 mL and 500 mL were detected. 78 Sensitivity of detection falls when effusions are bilateral. The volume of pleural effusions is generally underestimated in supine patients. 77 The signs produced by pleural effusions on supine radiographs are as follows:
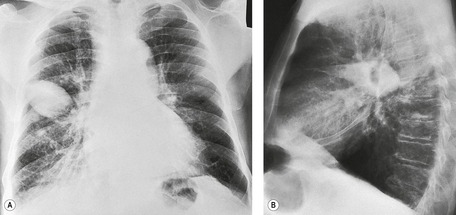
• Hazy, veil-like opacity of one hemithorax with preserved vascular shadows (Fig. 15.24). With smaller effusions, this veiling may occupy only the lower chest, making the lower half of the hemithorax more opaque than the upper, with a gradual transition to more normal lung density. 78
• Capping of the lung apex with a pleural shadow. 38 Some regard this as a relatively early sign explicable because the chest apex has a small capacity and is the most dependent part of the pleural space tangential to a frontal X-ray beam in a supine patient. 38 However, it seems that effusions have to be at least moderate (more than 500 mL) before they collect at the apex. 78 With the accumulation of more fluid a bandlike opacity develops and separates the lateral lung margin from the chest wall.
• Thickening of the minor fissure. 38
• Widening of the paraspinal soft tissues. 81
• Apparent elevation of the hemidiaphragm and reduced visibility of lower lobe vessels behind the diaphragm. 80
Hydropneumothorax may be recognized by the presence of a pleural line with increased density lateral to it in the pleural space. 82
CT of pleural fluid
In selected patients CT plays an important role in the assessment of pleural effusion83 for the following reasons.
• The configuration of the pleural opacity may allow a distinction between free and loculated fluid. When the effusion is loculated, CT can assess its extent and localization. If loculation is suspected on the basis of initial supine CT images, it may be confirmed by obtaining a further set of images in decubitus or prone positions.
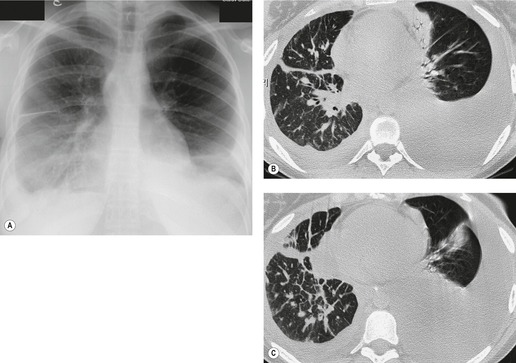
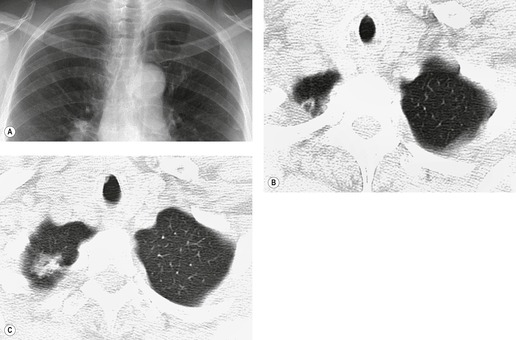
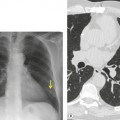
• CT is superior to the chest radiograph for differentiating between pleural and parenchymal disease85 (Fig. 15.25). Such a distinction is greatly helped by administration of intravenous contrast material, which facilitates identification of lung parenchyma and pleura. 86 Optimal CT detection of pleural enhancement may require a delay of up to 60 seconds after contrast administration.
• CT allows assessment of pleural morphology. Some appearances (irregular or nodular thickening and focal masses) are highly suggestive of malignancy (Fig. 15.26), and others (mild uniform thickening) of benignity. 87
• CT may allow identification of underlying lung disease (tumor, pneumonia, tuberculous lesion, abscess).
• It facilitates percutaneous biopsy of abnormal-appearing pleura (Fig. 15.26), and drainage of large or loculated fluid collections.
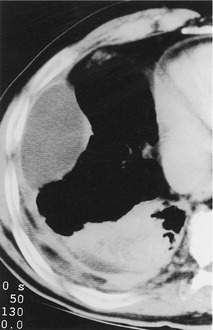
Although comparative studies have not been performed, CT is undoubtedly more sensitive in the detection of pleural fluid than an erect posteroanterior chest radiograph. Pleural effusion gives a homogeneous crescentic opacity in the most dependent part of the pleural cavity, usually in the midthorax. The lower CT attenuation of pleural fluid usually allows distinction from dependent atelectasis, pleural thickening, and masses (Fig. 15.26). 87 In general, the attenuation coefficient of pleural fluid does not allow reliable differentiation among exudate, transudate, and malignant effusion.88. and 89. However, hemothorax is usually recognizable on CT as a nonhomogeneous collection with CT attenuation subtly greater than water (Fig. 15.14). 90 The fat content of a chylothorax might be expected to produce low CT numbers, but the associated high protein levels usually result in attenuation values near water density, although occasionally modestly low numbers from −10 Hounsfied Units (HU) to −20 HU are recorded.91. and 92. Small amounts of pleural fluid can be difficult to distinguish from pleural thickening, a situation that may be clarified by repeat scanning in a changed position. 85
Analysis of the morphology of the pleura and extrapleural fat may help to differentiate between exudates and transudates. 93 In 59 exudative effusions, there was CT evidence of parietal pleural thickening in 36 and, of these, 20 patients also had concurrent thickening of the extrapleural fat. By contrast, thickening of the parietal pleura was demonstrated in only a single case with a transudative effusion. In a study of 211 pleural effusions by Arenas-Jimenez et al., 94 CT findings of loculation, pleural thickening, pleural nodules, and extrapleural fat of increased density were specific for exudative effusions. Multiple pleural nodules and nodular pleural thickening were seen only in malignant effusions.
Pleural fluid deep in the posterior and lateral costophrenic sulci must be distinguished from ascites, and a variety of signs have been described to differentiate pleural and ascitic fluid. Pleural fluid collecting between the diaphragmatic crus and the spine displaces the crus anterolaterally (Fig. 15.27), whereas ascitic fluid collects anterolateral to the crus and causes the opposite displacement.95. and 96. The interface between pleural fluid and liver or spleen is hazy (Fig. 15.27), 97 probably because of a partial volume effect from the obliquely sectioned diaphragm, in contrast to the sharp interface between ascitic fluid and intraabdominal organs. If the diaphragm can be identified, fluid inside the dome must be ascites and fluid on the outside must be pleural, provided the diaphragm is not inverted.96. and 98. Finally, ascitic fluid will be unable to reach the bare area over the posteromedial surface of the right lobe of the liver. Multiplanar reconstructions will usually definitively distinguish ascites from pleural fluid.
Sometimes the curvilinear opacity produced by atelectatic lung mimics the appearance of the diaphragm on noncontrast CT (Fig. 15.27). In general, atelectatic lung is thicker than the diaphragm, tapers laterally, and may be interrupted. 99 In addition, contiguous or multiplanar images usually show continuity with air-containing lung.99. and 100. On CT a loculated fluid collection usually produces a lenticular opacity with uniform, smooth inner walls. When the pleura encasing the loculation is thickened, or when it enhances with contrast, a split pleura sign is produced. 101 In addition, loculated fluid produces a mass effect with compression and distortion of adjacent lung. CT of loculated fluid is discussed in more detail in relation to empyema (p. 222).
Ultrasonography and pleural fluid
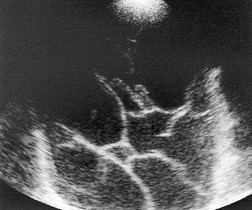
Ultrasonography is ideal for imaging pleural fluid, provided there is no intervening air-containing lung.7.9. and 84. Pleural fluid is commonly anechoic and delineated on its lung aspect by a sharp, highly echogenic line. 84 This echogenic line is due to high-amplitude reflections at the interface between pleural fluid and air-filled lung. Anechoic or hypoechoic effusions may be transudates or exudates.102. and 103. Echogenic pleural lesions can usually be distinguished from solid masses by imaging during respiratory maneuvers, when the effusion may change in shape84 or exhibit Doppler flow signal104. and 105. or swirling movement of finely echogenic debris. 106 Septations (Fig. 15.28) or fibrinous strands may be seen in complex effusions,107. and 108. and may move with breathing. The presence of septations does not preclude image-guided aspiration or drainage.
Ultrasonography is valuable in the evaluation and management of pleural fluid for several reasons:
• It is an excellent method for distinguishing solid from fluid pleural lesions, 109 using the signs described above (Fig. 15.29). However, there are occasional pitfalls in which fluid with abundant cellular or fibrinous content may be misclassified as solid. In one series three out of 90 pleural fluid collections (one empyema and two parapneumonic exudates) were misclassified as solid. 106 Conversely, hypoechoic solid lesions such as lymphoma or neurogenic tumors can be misinterpreted as fluid. 108
• It enables differentiation of peripheral lung lesions from pleural fluid110 and allows evaluation of their relative extent (Fig. 15.30). Findings that help in this assessment include the angle between the lesion and the chest wall and in consolidation the presence of vessels on Doppler examination and of airways as evidenced by air or fluid bronchograms. 84 In general, peripheral lung lesions make an acute angle with the chest wall, whereas with pleural lesions the angle is obtuse. Exceptions to this observation occur when peripheral lung lesions infiltrate the region of the adjacent visceral pleura.
• It may be helpful in evaluation of the opaque hemithorax. In this situation, the absence of air allows ultrasonography to identify the relative extent of pleural fluid, and of underlying pulmonary consolidation, mass, or abscess. 111
• It enables identification of pleural fluid in unusual sites such as in a subpulmonic location.
• It pinpoints localized fluid collections for aspiration, both diagnostic and therapeutic. 112 Indeed in instances where clinically guided thoracentesis has failed, ultrasonography is successful in the majority of cases. 113 Ultrasonography-guided thoracentesis appears to be associated with a lower risk of pneumothorax than blind thoracentesis, particularly when the effusion is small. 114 It is particularly helpful in intubated patients.115.116.117. and 118.
• It may identify areas of pleural thickening, raising suspicion of malignancy and enabling directed biopsy (Fig. 15.31).84. and 103.
• It can suggest the nature of an effusion. Transudates are typically anechoic and unaccompanied by pleural thickening. 103 Exudates seen with empyema, malignancy, parapneumonic effusions, and hemothorax are often, though not always, complex with diffuse or multifocal echogenicity, septation, or stranding.102.103. and 119. Effusions that are diffusely and homogeneously echogenic are commonly empyemas or caused by hemorrhage. 103
• It is helpful in identifying the causes of some effusions, both inside the chest (e.g. pneumonia) and outside, such as those caused by subphrenic or hepatic abscesses. 120
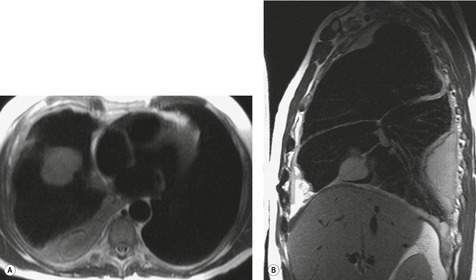
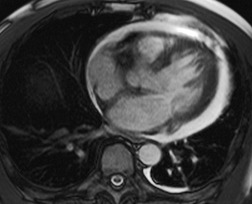
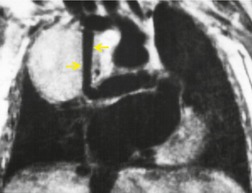
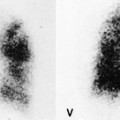
MRI and pleural fluid
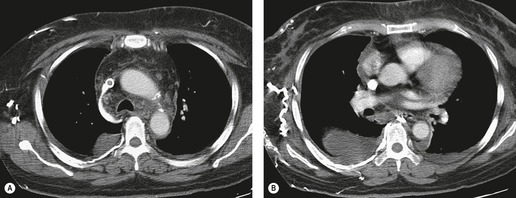
Apart from its role in staging of mesothelioma121 (Fig. 15.32), magnetic resonance imaging (MRI) has no significant role in investigation of pleural effusions. Pleural fluid has low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (Fig. 15.32) and gradient echo images (Fig. 15.33). The signal is often heterogeneous because motion within the effusion creates flow artifacts. Magnetic resonance (MR) signal characteristics can be helpful in identifying hemothorax,122. and 123. and pleural thickening or chest wall involvement (Fig. 15.32) but cannot otherwise be used to determine the character of pleural fluid.
Specific causes of pleural effusion
In this section, specific causes of pleural effusion are considered in detail. Effusions caused by pleuropulmonary infection, neoplasm, trauma, asbestos exposure, and collagen vascular diseases124 are discussed elsewhere. The major causes of pleural effusion are listed in Boxes 15.3 and 15.4.
Pregnancy-related pleural effusion
Pleural fluid may be detected both antenatally and in the immediate postnatal period. However, the prevalence of this finding is unclear. In two series which used chest radiographs to detect pleural effusion following normal vaginal delivery, the prevalence was 23%4 and 67%. 125 In 50 near-term women studied by ultrasonography, there was evidence of pleural effusion in six (12%) cases, 17 and fluid persisted into the postpartum period in three of these six patients. However, in another ultrasonographic study of 50 postpartum women, pleural effusion was found in only one patient who had preeclampsia. A thin (2–3 mm) layer of pleural fluid was identified on careful ultrasonographic examination in 28 of 47 pregnant women; 126 however, the same authors found pleural fluid in 28 (26%) of 107 healthy nonpregnant individuals. 127
Peripartum pleural effusions, when present, are most commonly small and bilateral. Other causes for pleural effusion associated with pregnancy may include infection, pulmonary thromboembolism, fluid overload, cardiomyopathy, preeclampsia, and the HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome.128. and 129.
Adjacent infection
Infectious processes in the upper abdomen commonly cause pleural effusions, which are usually sterile transudates. This happens particularly with subphrenic (and hepatic) abscesses but also occurs with other forms of upper abdominal suppuration such as perinephric or splenic abscess. 130
Subphrenic abscess
Subphrenic abscess most commonly follows upper abdominal surgery, particularly involving the stomach and spleen. 131 A significant proportion of cases, between 10% and 20%,131.132. and 133. are not directly related to surgery but follow perforation of a hollow viscus, pancreatitis, or trauma, and a small number are cryptogenic. A subphrenic abscess is commonly delayed to 1–3 weeks after surgery but may not develop until many months afterward. With abscesses in contact with the diaphragm there are nearly always changes on the plain chest radiograph. 132 About 80% of patients have a pleural effusion,131.132. and 134. which is usually small to moderate in size. Effusions are usually sterile, but occasionally they are empyemas. 135 Additional radiographic findings commonly include an elevated hemidiaphragm, basal collapse and consolidation, and an air–fluid level below the diaphragm. The diagnosis may be established by ultrasonography or CT. 136
Hepatic abscess
In a series of 53 patients with pyogenic liver abscesses the chest radiograph was abnormal in 53%. 137 The changes most commonly noted were basilar atelectasis and pneumonitis (44%), hemidiaphragm elevation (31%), and pleural effusion (20%). An air–fluid level was present below the hemidiaphragm in 7% of these patients. The possibility of a hepatic abscess (amebic or pyogenic) should be considered in all patients with an obscure right-sided exudative pleural effusion, 138 particularly if fever, anorexia, and abdominal pain are present. Ultrasonography or CT may be pivotal in diagnosis and management.
Cardiovascular disease
Heart failure (Box 15.7)
In clinical practice heart failure is probably the most common cause of a transudative pleural effusion; the volume of pleural fluid may range from barely detectable to considerable. 27 It is generally thought that pleural effusions in heart failure are more common and larger on the right, and indeed some authors have suggested that isolated left pleural effusion in heart failure may indicate an additional disease process such as pulmonary embolism. 139 However, a review of autopsy and clinical series shows that the excess of right-sided effusions in heart failure is not marked: 26% right sided, 16% left sided, 59% bilateral.140.141.142.143.144.145. and 146. The possibility of pulmonary infarction should always be considered in patients with heart failure who develop unexplained pleural effusions.145. and 146.
Box 15.7
• Usually require a combination of right and left heart failure
• May develop or increase as pulmonary edema resolves
• Usually transudative
• Most commonly bilateral, right larger than left
Two unusually distributed forms of fluid collection occur particularly in heart failure: fissural loculation forming pseudotumors (see Fig. 15.17), and lamellar ‘effusion’ (see Fig. 15.23).
The mechanism responsible for the development of pleural effusion in heart failure is still debated. Effusions often develop or increase when pulmonary edema is resolving, Most authors have suggested that a mixture of left and right heart failure is necessary. A much quoted experiment conducted in dogs147 showed that elevation of right-sided pressures caused a greater accumulation of pleural fluid than elevation of left-sided pressures, but that an increase of right- and left-sided pressures together was the most effective. On the other hand, a study of humans in heart failure following myocardial infarction showed that the presence of an effusion correlated much better with elevated left atrial pressure than with elevated right-sided pressures, 148 an observation that fits better with the low prevalence of pleural effusions in cor pulmonale or pulmonary hypertension.148. and 149. On balance it seems likely that edema fluid in the lung interstitium moves across the nontight mesothelial barrier of the visceral pleura into the pleural space along an interstitial–pleural pressure gradient. 6
Cardiac surgery
Left-sided pleural effusion occurs in up to 85% of patients undergoing open coronary artery bypass (CABG) surgery. 150 These are usually small, but about 10% of patients have large effusions. 151 Effusions occurring within 1 month of CABG tend to be bloody exudates, often associated with eosinophilia, while those occurring later are commonly lymphocytic, and are sometimes associated with visceral pleural thickening and trapped lung or rounded atelectasis.150. and 151. The early effusions have been ascribed to left ventricular failure combined with impaired drainage from the left pleural space following internal mammary artery dissection and pleurotomy. 152 Differential diagnosis of pleural effusion occurring after CABG must include heart failure, pulmonary embolism, 153 chylothorax,154. and 155. and postcardiac injury syndrome. 156 CABG performed with cardiopulmonary bypass has been associated with a relatively low risk of pulmonary embolism, because anticoagulation is routinely used. However, anticoagulation is not used with off-pump bypass surgery, which may therefore be associated with an increased prevalence of pulmonary embolism. 157
Post-cardiac injury syndrome
Post-cardiac injury syndrome follows a variety of myocardial and pericardial injuries, and is most commonly seen after myocardial infarction (Dressler syndrome) 158 or cardiac surgery (postpericardiotomy syndrome). 159 Other causes are described, including closed chest trauma, 6 coronary angioplasty and stenting, 160 and implantation of pacemakers or defibrillators.161.162. and 163. The syndrome is characterized by fever, pleuritis, pneumonitis, and pericarditis days or weeks after the precipitating event and often runs an intermittent course. Study of the condition has been hampered by lack of a diagnostic test and by clinical similarities with pneumonia, extension of myocardial infarction, heart failure, and pulmonary embolism, so that this condition remains a diagnosis of exclusion. The prevalence of postcardiac injury syndrome is probably about 5% following myocardial infarction158. and 164. and somewhat higher after cardiac surgery. 165 An incidence of 31% was reported in 161 patients undergoing surgery for the Wolff–Parkinson–White syndrome. 156 In one series the syndrome developed on average 20 days after cardiac injury (range 2–86 days). 166 The most common clinical features were pleuritic chest pain (91%), fever, pericardial rub, dyspnea, and pleural rub. Nearly all the patients had a high erythrocyte sedimentation rate, and 50% had leukocytosis. The postcardiac injury syndrome is usually self-limiting although persistence of pleural fluid157 with consequent fibrosis has been described. 167 Though the syndrome responds to anti-inflammatory treatments, relapse may follow drug withdrawal. 165
The chest radiograph is abnormal in more than 90% of patients. The chief findings are pleural effusion, consolidation, and a large heart. Pleural effusions were present in 81% of patients in four combined series,158.166.168. and 169. with unilateral and bilateral effusions being equally common. When unilateral, effusions are more common on the left than on the right. 166 About one-half to three-quarters of the patients have consolidation, which is usually unilateral (left more than right), and about half have a large cardiac silhouette. The diagnosis should be suspected in the appropriate clinical setting if there is pleural effusion(s) and consolidation, particularly if the cardiac silhouette is enlarged and there is no evidence of heart failure. 170 Echocardiography establishes the pericardial nature of the cardiac enlargement. The pleural fluid is a serosanguineous or bloody exudate. 166
Pericardial disease
Pericardial disease, both inflammatory and noninflammatory, may be associated with pleural effusions in a variety of conditions including congestive heart failure, constrictive pericarditis, metastatic malignancy, collagen vascular disease, postcardiac injury syndrome and infection. Weiss and Spodick171 assessed a series of 35 patients with pleural effusions and a variety of pericardial diseases and found that effusions were solely left sided in 60% and predominantly left sided in 71% (9% were right predominant and 20% equal bilaterally) (Fig. 15.34).
Superior vena cava syndrome
Occlusion of the superior vena cava might be expected to predispose to the formation of pleural effusion147 because it increases the parietal pleural hydrostatic pressure, thereby increasing fluid filtration from parietal pleural capillaries, and it reduces lymphatic flow from the thoracic duct and right bronchomediastinal trunk. 172 However, pleural effusion secondary to vena caval obstruction is uncommon.173. and 174. Chylothorax should be considered as a potential cause of effusion in those with obstruction of superior vena cava or brachiocephalic veins.175. and 176. The converse situation in which a large encapsulated pleural effusion causes obstruction of the superior vena cava has also been reported. 59
Pulmonary embolism
Pleural effusion is common in pulmonary embolism, occurring in a quarter to half of the patients.177.178. and 179. Effusions are more likely to occur with pulmonary infarction but can be seen without it.177. and 180. In a recent study of 230 patients with pulmonary embolism, effusions were seen in 32% by chest radiograph and 47% by CT. 181 Effusions were generally small (90% occupied less than one-third of the hemithorax) and were unilateral in 85% of cases. Loculation was identified on CT in 21% of cases, and appeared to be related to delay in diagnosis of pulmonary embolism. The presence of pleural fluid was unrelated to the presence of infarction. The effusions usually disappear within about a week (72% disappearing by 7 days), 177 but effusions accompanied by pulmonary consolidation may take longer to clear. Enlargement of a pleural effusion related to pulmonary embolism should lead to consideration of a complication such as hemothorax or infection. 182
Ascitic effusion
Ascitic fluid of any cause may be associated with a pleural effusion. The mechanism of pleural effusion formation in the majority of these patients seems to be transdiaphragmatic passage of fluid. The association of ascitic and pleural fluid is well recognized with hepatic cirrhosis, peritoneal dialysis, ovarian hyperstimulation syndrome,183. and 184. intraperitoneal dextran, 185 and Meigs syndrome.
Hepatic hydrothorax
The term ‘hepatic hydrothorax’ has been given to the accumulation of a transudative effusion in cirrhotic patients in whom cardiac, pulmonary, and primary pleural causes have been excluded.186. and 187. A prevalence varying between 0.4%188 and 12%189 is recorded in various series, a range that reflects the differing severity of the underlying liver disease. In the context of an intensive care unit, hepatic hydrothorax was an underlying cause in five out of 62 (8%) patients with pleural effusion. 190 Effusions may be small to massive, and they show a predilection for the right side (see Fig. 15.12). Thus, in 55 cases from six series, 60% were right sided, 22% left sided, and 18% bilateral.189.191.192.193.194. and 195. In one series that looked at cirrhosis with isolated left pleural effusion, 18% of effusions were found to be tuberculous. 196 The authors concluded that isolated left-sided effusions in the context of cirrhosis should be fully investigated and not assumed to be the result of liver disease per se. 196
Although raised lymphatic pressure, azygos vein hypertension, and hypoalbuminemia play a part in generating cirrhotic effusions, 197 evidence indicates that transdiaphragmatic passage of fluid is the most important mechanism. 193 Ascites is a common, but not universal, finding in hepatic hydrothorax.189. and 198. Conversely, not all patients with cirrhotic ascites have pleural effusions. In one series of 330 patients with hepatic ascites only 5.5% had pleural effusions on plain chest radiography. 192 Transfer of fluid from the peritoneal cavity to the chest has been clearly demonstrated by studies in which blue dye, India ink, and radionuclide-labeled albumin have been introduced into ascitic fluid.191. and 192. It is also well recognized that air in the peritoneal cavity can enter the pleural cavity, usually on the right.192.193. and 199. Furthermore, defects have been identified in the diaphragm at postmortem examination, 192 at thoracotomy, and thoracoscopy.192. and 200. Fluid and air have been seen to pass through these defects. 192 The defects are usually found in the tendinous part of the diaphragm and are probably tears produced by the stretching that occurs in the presence of ascites. 193 Localized muscle thinning where vessels pass through the diaphragm may be another predisposing factor. The pathologic features of these defects were elegantly demonstrated by Lieberman and Peters. 193 Some of the defects were still closed by peritoneum and appeared as fluid-filled blebs on the pleural side of the diaphragm, whereas in others the peritoneal membrane had ruptured, leaving a hole. The structure of the blebs, their possible sudden rupture, and their later occlusion by adhesions probably explains why many patients with ascites have no pleural effusion192 and why effusions may develop or resolve suddenly.192.193. and 195. The right-sided predilection of effusions in ascites may be related to the greater exposure of the tendinous diaphragm on the right, where it is not covered by the heart. Alternatively, it could be due to the action of the liver as a one-way valve, allowing entry of ascitic fluid into the chest on inspiration, but preventing its return to the abdomen on expiration. Drainage of hepatic hydrothorax via chest tube is not usually recommended because the pleural fluid is promptly replenished from the abdomen. Treatment of hepatic hydrothorax is based on management of the underlying ascites, and transjugular portosystemic shunting is usually effective.187.201. and 202.
A number of papers have described pleural effusion occurring with hepatitis. 6 Potential etiologies of effusion in this context include hypoproteinemia, sympathetic effusion related to subdiaphragmatic inflammation, and hepatic hydrothorax.
Meigs syndrome
The earliest descriptions of Meigs syndrome are those of Salmon203 in 1934 and Meigs and Cass in 1937. 204 The syndrome has four components: ascites, pleural effusion, ovarian fibroma, and resolution of ascites and hydrothorax with removal of the tumor. The definition was later extended205. and 206. to include other ovarian tumors: theca cell, granulosa cell, and Brenner tumors. More recently there has been a tendency to include all ovarian and uterine neoplasms associated with benign effusions. 207 In particular, there have been numerous case reports of uterine leiomyomata causing a Meigs-like syndrome.208.209.210.211. and 212. Some authors call this extended syndrome the atypical (pseudo) Meigs syndrome.213. and 214. Majzlin and Stevens206 reviewed 128 cases of the more limited syndrome and found ovarian fibroma in 82%, theca cell tumor in 9.4%, granulosa cell tumor in 4.7%, and Brenner tumor in 1.6%. The fluid in the chest and abdomen was the same and usually clear, amber-colored transudates. However, various appearances of the effusions are described, including hemorrhagic. 215 Pleural effusions are more commonly right sided with a frequency of 65% on the right, 10% on the left, and 22% bilaterally (Fig. 15.35). 206 Meigs syndrome is uncommon; in one series, out of 283 patients with ovarian fibromas, 51 had ascites but only two had pleural effusions as well. 216 The source of the ascites is not clearly established, but evidence suggests that it is produced by transudation from the surface of the larger tumors, possibly resulting from vascular compromise. 216 Once formed, ascitic fluid almost certainly enters the chest through diaphragmatic defects, as discussed above.
The importance of Meigs syndrome lies in the fact that neither ascites nor pleural effusion necessarily indicates that a pelvic mass is malignant and has metastasized. However, Meigs-type syndromes have been described with malignant tumors. 217
Pancreatic disease
Pleural effusion occurs in both acute and chronic pancreatitis, 138 and several mechanisms may be pathogenetically important. 6
The prevalence of pleural effusion in acute pancreatitis is about 10–20% but may range from 4%218 to 38%. 219 The presence of an effusion suggests severe or even necrotizing pancreatitis.220.221. and 222. Because of the close relation of the pancreas, particularly the tail, to the left hemidiaphragm, effusions are usually left sided (70%) or bilateral (15%)219.221. and 223. and are thought to be lymphatic or sympathetic in origin. 224 There may be additional elevation of the hemidiaphragm together with basal consolidation. The clinical picture is usually dominated by abdominal symptoms, although occasionally the patient has dyspnea or pleuritic chest pain. 138 The effusions usually resolve completely. Pancreatic effusions are exudates and often hemorrhagic. The amylase levels of the pleural fluid are high and may exceed those in the serum. Pleural amylase values remain elevated even after serum levels have returned to normal values. 225 An elevated amylase level in pleural fluid is not, however, a specific indicator of pancreatitis and may be seen with esophageal perforation and occasionally with pleural malignancy. 138
Effusions associated with chronic pancreatitis are often large and recurrent,224.226.227. and 228. and in contrast to acute pancreatitis, chest symptoms usually dominate the clinical picture. 138 The pathogenesis of such effusions is thought to be posterior ductal rupture, allowing pancreatic fluid to move retroperitoneally into the chest via the aortic and esophageal hiatus (Fig. 15.36).229.230. and 231. Ninety-six cases were comprehensively reviewed by Rockey and Cello224 The patients were characteristically young to middle-aged men with a history of alcohol misuse (80%). At presentation 94% of patients had chest symptoms (67% without abdominal complaints) and only 52% gave a history of pancreatitis. Plain chest radiographs showed pleural effusions, often large with a left-sided predominance (left 67%, right 19%, bilateral 14%). Since pleural fluid had drained directly from the pancreatic duct, pleural fluid amylase levels were dramatically high.
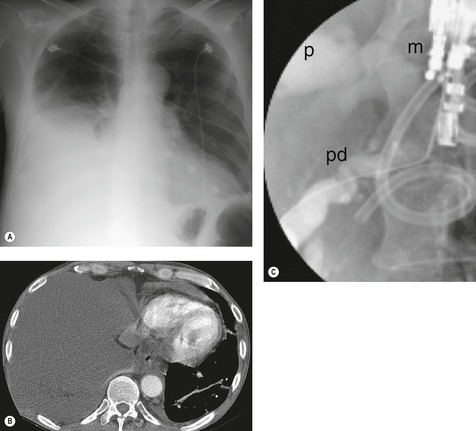
On CT, the majority (79%) of patients demonstrate pancreatic pseudocysts and a fistulous track is shown in about 40% of patients.231. and 232. In the remaining patients the CT scan is normal or shows changes of pancreatitis. Endoscopic retrograde pancreatography (ERCP) shows a fistulous track229. and 233. in 59% of patients (Fig. 15.36), and when results from ERCP and CT are combined, the track is shown in 70% of patients, and cysts and/or chronic pancreatitis in 25%. Magnetic resonance pancreatography appears to be an effective way of demonstrating pancreato-pleural fistulae in these patients.234.235.236. and 237. Treatment options include percutaneous drainage of pseudocysts, pancreatic stenting, 238 somatostatin treatment, 239 or excision of the pancreaticopleural fistula. 224 Pleural effusions caused by chronic pancreatitis may cause gross pleural thickening requiring decortication. 138
Renal disease
Uremic pleurisy and hemodialysis
A fibrinous pleuritis is common in uremia. 241 It may develop despite hemodialysis242 and is often accompanied by pericarditis. Patients may be asymptomatic or have chest pain and fever.242. and 243. The effusions are exudates243 and are commonly bloody. 244 In one series 79% were unilateral. 242 The effusions may be small or large. In patients who continue to undergo hemodialysis they often subside over weeks. Sometimes, however, fibrous pleural thickening ensues and decortication is required.245. and 246.
Urinothorax
Urinothorax is an unusual condition, usually related to dissection of urine along the posterior pararenal space, which is in contact with the posterior pleural space, allowing urine to escape into the pleural space. 247 It is most commonly iatrogenic related to renal biopsy247 or nephrostomy, 248 but it may also result from urinary tract obstruction.249.250.251.252. and 253. Other causes include blunt trauma. 254 The effusions are usually unilateral and on the same side as the obstructed or traumatized kidney. 130
In the appropriate clinical setting, a low-pH transudate with pleural fluid creatinine higher than serum creatinine establishes the diagnosis. 6 The pleural collections subside quite quickly following appropriate management of underlying urinomas or hydronephrosis.
Other renal diseases
Transudative effusions develop in about 20% of patients with nephrotic syndrome because of the associated hypoalbuminemia. 6 The effusions tend to be bilateral and are commonly subpulmonic and recurrent. 255 If the effusion is unilateral6 exudative or hemorrhagic, pulmonary embolism should be suspected. 256 Renal257 and perirenal130 infections are rare causes of ipsilateral sterile exudative pleural effusion, thought to be sympathetic in origin.
Peritoneal dialysis
Hydrothorax can develop during peritoneal dialysis, usually due to direct transit of dialysate across diaphragmatic defects.258. and 259. Nomoto et al. 260 reported that the prevalence of this complication was 1.6%. About 50% of such effusions develop within 30 days of the initiation of dialysis, but effusions may be delayed by a year or more. Like ascites-related pleural effusions, those caused by dialysis are usually on the right, 130 although a few have been bilateral or left sided. 261 Elevated levels of glucose confirm the presence of dialysate in the pleural effusion. 262 The mechanism is believed to be direct transfer of fluid from the peritoneal cavity into the pleural space through diaphragmatic defects. 5 The effusions respond to cessation of dialysis, but may recur when dialysis is restarted, and pleurodesis may be required. 5
Splenic disease
Splenic disease is an uncommon cause of left-sided pleural effusion. Splenic abscess, most commonly caused by infective endocarditis, is associated with a left pleural effusion in 20%–50% of patients and may be accompanied by basal collapse and consolidation and hemidiaphragm elevation.263.264. and 265. Left pleural effusion is also described associated with splenic infarction266 and splenic hematoma. 267
Abdominal surgery
Pleural effusions following abdominal surgery are common; half of the 200 patients in one series had evidence of pleural fluid on chest radiographs obtained within 3 days of surgery. 268 Predisposing factors included upper rather than lower abdominal surgery and postoperative atelectasis. All but one of the effusions settled without complications. The benign nature of early effusions was confirmed in a prospective study of 128 patients having upper abdominal surgery, 70% of whom developed unilateral (37%) or bilateral (63%) effusions. 269 These small early effusions should be distinguished from larger ones that develop later, since these are more commonly clinically significant, suggesting a complication such as subphrenic abscess. 131
The development of a unilateral effusion in patients with a chronic traumatic diaphragmatic hernia suggests strangulation and infarction of the contained bowel. 270
Radiation
Pleural thickening and effusion are recognized complications of radiation therapy, usually due to radiation pleuritis.271. and 272. In one series of 200 patients treated for carcinoma of the breast, 11 developed pleural effusions within 6 months: all had associated radiation pneumonitis. The effusions were mostly small, and decreased or disappeared over months or years. 273 Suggested criteria for the diagnosis of radiation pleuritis are effusion within 6 months of completing radiation therapy, coexisting radiation pneumonitis, and spontaneous resolution. 273 Reaccumulation or rapid increase in the pleural effusion suggests metastasis. Effusions may also rarely occur as a late complication of radiation therapy, probably secondary to lymphatic or venous obstruction from mediastinal fibrosis. 6
Drugs and the pleura
A number of drugs may cause pleural effusions or pleural thickening. The useful website Pneumotox online (www.pneumotox.com) lists over 50 drugs which may cause pleural effusion, pleural thickening, or hemothorax. The most common drugs are listed in Box 15.8 and all are considered in this section apart from those associated with systemic lupus erythematosus. Several comprehensive reviews of drug-induced pleural disease are available.274. and 275.
Box 15.8
Drugs causing systemic lupus erythematosus
• Hydralazine
• Isoniazid
• Phenytoin
• Procainamide
• Others (rarely)
Cytotoxic drugs
• Bleomycin
• Busulfan
• Methotrexate
• Mitomycin
• Procarbazine
• Interleukin-2
• All-trans-retinoic acid (ATRA)
Antibacterial drugs
• Nitrofurantoin
Antimigraine drugs
• Ergotamine
• Methysergide
Antiarrhythmic drugs
• Amiodarone
Antithyroid drugs
• Propylthiouracil
Skeletal muscle relaxants
• Dantrolene
Vasodilator antihypertensives
• Minoxidil
Dopaminergic receptor stimulants
• Bromocriptine
β-Adrenergic blockers
• Acebutolol
• Practolol
• Gonadotrophin
Antineoplastic drugs
Pleural changes are described with all types of methotrexate regimens: intermittent high dose, 276 intermittent low dose, 277 and maintenance. 278Urban and colleagues276 described a 9% prevalence of pleuritis with a high-dose regimen that produced thickening of fissures on the radiograph but no free fluid. In another series a 4% frequency of pleuritic pain and a 1% frequency of pleural effusion were described. 277 An intermittent low-dose regimen gave a 4% prevalence of pleuritis, but only about 1% had radiographic evidence of pleural effusion. 276 Maintenance therapy may be associated with pulmonary eosinophilia in which dominant parenchymal shadowing is occasionally accompanied by a small pleural effusion. 278 Treatment with procarbazine has been associated with bilateral interstitial shadowing, blood eosinophilia and pleural effusions. 279 Pleural effusions have also been described with mitomycin, 280 busulfan, 281 and bleomycin.282. and 283.
Interleukin-2, used for its cytotoxic activity in renal cell carcinoma and melanoma, can produce a capillary leak syndrome characterized by pulmonary edema and pleural effusions.284. and 285. Uncommonly, pleural effusions are isolated. 286 ATRA, used in treatment of acute promyelocytic leukemia, has also been found to cause effusions, in addition to lung edema. 287
Antibacterial drugs
Two syndromes are recognized with nitrofurantoin toxicity: an acute syndrome that develops after hours or days and is probably immunologically based, because the patient usually has taken nitrofurantoin in the past and has eosinophilia. The chronic syndrome is less commonly associated with eosinophilia and appears months or years after therapy begins. 165 In one large series 35% of acute reactions were accompanied by parenchymal opacity and pleural effusions, while isolated pleural effusions developed in a further 8%. In the chronic syndrome there was a 7% prevalence of effusions, which were never the sole manifestation. 288
Antimigraine drugs
Ergot derivatives, such as methysergide and ergotamine, are known to cause pleural and parenchymal disease.289. and 290. More than 30 cases of methysergide-induced pleuropulmonary disease have been reported since the first report in 1966. 291 The syndrome may appear between 1 month and 6 years after beginning therapy. 165 Radiography shows unilateral or bilateral pleural thickening and effusions.292.293. and 294. Pleural effusions sometimes appear to be loculated. 295 Localized pleural and subpleural fibrosis can stimulate a mass lesion. 292 Ergotamine has been implicated in causing unilateral pleural thickening with effusion, 296 and ergonovine may have the same effect. 295 Pleural thickening with concurrent pericardial fibrosis has also been reported following treatment with ergotamine. 297
Antiarrhythmic drugs
In addition to parenchymal opacities, amiodarone may occasionally cause pleural thickening or effusions.298.299.300. and 301. On average the onset is 6 months after the start of treatment, but the delay ranges from 1 month to several years and the changes are rarely seen if the dose is less than 400 mg/day. The prevalence varies between 1% and 6%. 302 All patients with pleural abnormality have had parenchymal involvement. 299
Skeletal muscle relaxants
Dantrolene may cause chronic symptomatic pleural effusion, which can be unilateral and rich in eosinophils. 303 Symptomatic improvement occurs within days after the drug is stopped, but radiographic clearing may take months.
Dopaminergic receptor stimulants
Bromocriptine probably causes pleural changes in 2–5% of patients receiving the drug for long-term treatment of Parkinson disease. 304 All patients have been male. Symptoms consist of dyspnea, cough, and pleuritic pain, usually developing 1–2 years after the beginning of treatment. Radiographic findings are pleural thickening and effusion, either unilateral or bilateral. 304 Interstitial lung opacity may be present. 305 Although symptoms may abate after withdrawal of bromocriptine, pleural thickening generally persists.290. and 304.
β-Adrenergic blockers
Now largely of historical interest only, since the withdrawal of practolol from the market in 1976. There is a single case report of acebutolol causing subtle visceral pleural thickening (and associated pulmonary fibrosis) which was visible only on CT. 306
Ovarian hyperstimulation syndrome
Occasionally gonadotrophins administered for infertility secondary to ovulation failure cause a syndrome characterized by abdominal pain and distension, cystic ovarian enlargement, and ascites with pleural effusion.183. and 184. The ovarian hyperstimulation syndrome is associated with high circulating levels of estrogen but the cause of ascites in this condition is unclear. Pleural effusion is usually secondary to the ascites, but patients with isolated pleural effusion and no ascites are recorded.22.307. and 308. Although usually bilateral (Fig. 15.37), the effusions in this condition may sometimes be unilateral and massive.309. and 310. The chest radiograph commonly shows decreased lung volumes due to the accompanying ascites (Fig. 15.37).
Miscellaneous causes of pleural effusion
Myxedema
Pleural effusions are described in myxedema, 311 but whether they are more commonly transudates or exudates is not clear312 possibly because they have borderline characteristics. 313 In one series of patients with myxedema, about half had a pleural effusion, 314 and these were usually accompanied by pericardial effusions although isolated pleural effusion is recorded. 315 In another series of 128 patients with hypothyroidism, 28 had a pleural effusion of which 79% were nonhypothyroid effusions, 4% were associated with pericarditis, and 18% were diagnosed as hypothyroid effusions by exclusion. 313 Effusions were as commonly bilateral as unilateral and occupied less than one-third of the hemithorax. The presence and size of the effusion were not correlated with the degree of hypothyroidism. 313 Effusions disappear with treatment of the myxedema. 315
Pleural effusion with yellow nails and primary lymphedema (Box 15.9)
The association between yellow nails and primary lymphedema was first described in 1964, 316 and in the same year the association between pleural effusion and primary lymphedema was recorded. 317 Two years after these accounts the triad of yellow nails, pleural effusion, and primary lymphedema was reported, 318 and by 1986 nearly 100 cases were on record. 319 The nails of fingers and toes are yellow and dystrophic320 and subject to both onycholysis and infection. Lymphedema typically affects the lower legs and is mild. 321 The pleural effusions may be unilateral or bilateral, small or large,320. and 322. and are characteristically chronic and persistent. 319 Pleural thickening is common. The exudates are rich in protein and lymphocytes, but since there is no reflux from the thoracic duct into the lungs, they are not chylous. Bronchiectasis is usually present and often symptomatic. 323 In a review of 41 patients with the syndrome, 46% had pleural effusions, 44% had bronchiectasis, 41% had chronic sinusitis, and 22% had recurrent pneumonia. 324 Other, less common features are erysipelas and hypogammaglobulinemia. 319 The syndrome may be familial, 325 and may be found in patients with rheumatoid arthritis. 326
Box 15.9
• Triad of yellow nails, pleural effusion, and lymphedema
• Unilateral or bilateral effusions are often chronic
• Bronchiectasis is common
• Sinusitis may also occur
In a review of 97 patients the male/female ratio was 1 : 1.6 and the median age at presentation was 40 years. 319 However, the age at presentation and the severity of the manifestations vary widely. At presentation only about half of the patients have the classic triad. About a third of patients have respiratory symptoms at this time, often preceded by a long history of sinopulmonary infections. 6 All elements of the triad are thought to result from lymphatic hypoplasia,5.6.327. and 328. but the mechanism of the bronchiectasis and sinusitis is unclear. The effusions may require pleurectomy or chemical pleurodesis.317.319.329. and 330.
Familial Mediterranean fever
Familial Mediterranean fever is a rare, autosomal recessive disorder characterized by recurrent attacks of abdominal pain, arthralgia, and pleuritic pain accompanied by fever. It has several synonyms, including periodic disease, periodic fever, recurrent polyserositis, and familial paroxysmal polyserositis. 331 It occurs almost exclusively in non-Ashkenazic Jews, Armenians, Turks, and Arabs. The disease develops in the first decade in 50% of those affected and before the age of 20 years in 75% (range 1–40 years). Males outnumber females 2 : 1. 332 The most common presentation is with paroxysmal, recurrent peritonitis and fever lasting 1–4 days, which often lead to unnecessary laparotomy. The peritonitis is commonly accompanied by pleurisy, which is often overlooked. When first examined between 13% and 40% of patients have pleuritis.332.333. and 334. The prognosis is good unless renal amyloidosis develops.
Atelectasis, trapped lung, and pneumothorax ex vacuo
If a portion of lung is reduced in volume and prevented from reexpanding either by bronchial obstruction or by a postinflammatory visceral pleural peel (‘trapped lung’), the pleural pressure is reduced. This fall in pressure may disturb the balance of forces affecting pleural fluid formation and absorption, leading to the accumulation of pleural effusion. In such cases, the pleural pressure is likely to be negative rather than positive, as is usually the case with pleural effusions.338. and 339.
Thoracentesis or chest drainage in patients with trapped lung or obstructive atelectasis is likely to lead to pneumothorax, as the evacuated fluid is replaced by air (Fig. 15.38). This phenomenon is sometimes called pneumothorax ex vacuo, or negative pressure pneumothorax. Following thoracentesis, fluid usually reaccumulates rapidly to its previous level, replacing any air. There are scattered reports of pneumothorax ex vacuo occurring without any chest intervention in patients with obstructive lung collapse,340.341. and 342. but this is very rare. 5 Trapped lung may be suspected in patients with chronic stable effusions following prior pleural inflammation, particularly if a pneumothorax is seen after thoracentesis. The pneumothorax is usually small, and often confined to the area of collapse; the thickened pleura is often visible. Etiologies may include previous CABG, radiation, pleural infection, or malignant pleural thickening. 343 Diagnostic pneumothorax has been recommended to visualize the thickened pleura and establish this diagnosis,5. and 343. but is usually unnecessary, as the thickened visceral pleura is quite easily seen on CT, and pleural manometry will confirm the diagnosis.338. and 339. The presence of rounded atelectasis may be a clue to trapped lung. Aspiration or drainage of pneumothorax or fluid related to trapped lung is not indicated; the only available treatment would be surgical decortication.
CHYLOTHORAX
A chylothorax contains fluid that is largely chyle (lymph of intestinal origin). Because chyle usually contains suspended fat in the form of chylomicrons, chylothorax fluid is milky. A chylothorax should be distinguished from other milky effusions, particularly empyema and pseudochylothorax, which are discussed later. Before the causes of chylothorax are discussed, the anatomy of the thoracic duct and its tributaries and the physiology of chyle are briefly reviewed.
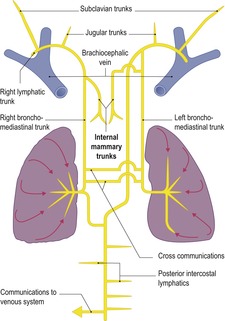
Anatomy of the thoracic duct and its tributaries
The thoracic duct connects the cisterna chyli to the great veins in the root of the neck and transports all of the body lymph except that from most of the lungs and the right upper quadrant of the body (Fig. 15.39).344.345.346.347. and 348. The cisterna chyli is formed by the fusion of the two lumbar lymphatic trunks346 and lies in front of the T12 to L2 vertebral bodies. The thoracic duct arises from the cisterna chyli. It is 2–8 mm in diameter and valved, particularly in its upper half, 346 which effectively prevents retrograde flow. 349 Although the thoracic duct is thought of as a single structure, it is commonly multiple in part of its course350 and may consist of up to eight separate channels. 346 Indeed, in one study, multiple ducts were more common than single ones and at the level of the diaphragms, where the duct is commonly ligated therapeutically, about a third were double. 349 The duct or ducts pass up from the cisterna behind the median arcuate ligament and ascend on the anterior aspect of the vertebral bodies and right intercostal arteries between the azygos vein and aorta. At the level of T6 the thoracic duct crosses to the left of the spine351 and ascends along the lateral aspect of the esophagus behind the aorta and left subclavian artery. Having reached the neck, it arches forward across the subclavian artery and inserts close to the junction of the left internal jugular and subclavian veins. The number of channels and the site of insertion of the terminal thoracic duct vary. Near its termination the thoracic duct receives the left bronchomediastinal trunk, which drains the left lung, the left jugular trunk, and the left subclavian trunk (Fig. 15.39). Any or all of these vessels may end separately in the great veins. On the right side is a right lymphatic duct that receives the right jugular, right subclavian, right internal mammary, and right bronchomediastinal trunk. The right bronchomediastinal trunk also receives communications from the left trunk (through which it commonly drains the caudad half of the left lung) and from the thoracic duct via the right posterior intercostal lymphatics (Fig. 15.39).
Knowledge of thoracic duct anatomy is clinically important for the following reasons:
• Immediately after thoracic duct rupture, chyle commonly collects in the mediastinum and may cause mediastinal swelling. 352 Eventually, however, it bursts through into the pleural space, after a delay that varies from days to months.
• Because the thoracic duct crosses from right to left in the middorsal region, chylothorax tends to be right sided with low chest trauma and left sided with trauma high in the chest.
• The thoracic duct is closely related to the aortic arch and mid-esophagus, and surgery to these structures is more likely to cause chylothorax than other cardiothoracic operations.
• Obstruction to the thoracic duct per se does not result in a chylothorax because of the duct’s extensive lymphatic and venous communications. 353 This means that it can be tied off therapeutically without adverse consequences, 354 and also implies that obstruction by neoplasm or other process can cause chylothorax only if it leads to thoracic duct rupture.349. and 355.
Mechanisms of chylothorax formation
Direct leakage of chyle from the thoracic duct occurs with trauma (often iatrogenic) or neoplastic involvement. Chyle will often accumulate within the posterior mediastinum (chyloma) before the mediastinal pleura is breached and chyle spills over into the pleural cavity (Fig. 15.40). 356
Thoracic duct obstruction, mediastinal lymphangiomas, or lymphangiectasia can cause extensive parietal pleural collaterals, and these may rupture, allowing seepage of chyle from the parietal pleura (Fig. 15.41). The degree and type of collateral formation vary depending on the richness of an individual’s lymphatic–lymphatic and lymphatic–venous connections. Thoracic duct obstruction may also result in reflux of chyle into the lungs. Chyle does not normally gain access to the lungs or visceral pleura because both the intrapulmonary lymphatics and the thoracic duct have valves. 357 Valvular incompetence or increased lymphatic pressure may however allow chyle to reflux into the lungs and to ooze from the visceral pleura over the lung surface (Fig. 15.41). The development of a ‘lymphangitic’ radiographic pattern in the lungs and a chylothorax has been described after obstruction of the thoracic duct and the right bronchomediastinal trunk. 176 The ‘lymphangitic’ pattern in these cases almost certainly represents dilated lung lymphatics distended by chylous reflux (Fig. 15.41).
As might be expected, obstruction of the superior vena cava or of both brachiocephalic veins may produce a chylothorax, since this compromises the drainage of both the thoracic duct and potential anastomotic channels.175.176.358.359. and 360. In an experimental study by Fossum and Birchard, 361 ligation of the superior vena cava below the azygos vein resulted in chylothorax in seven of 10 dogs, but ligation of the thoracic duct did not produce chylothorax.
Physiology of chyle
Lymph flowing up the thoracic duct is derived principally from the gut (60%) and to a lesser extent from the liver (35%) and the peripheral lymphatics (5%).55.347. and 362. About 1.5–2.5 L/day is produced.55. and 363. It flows upward because of contractions of the duct wall, adjacent arterial pulsations, gut contractions, the pressure gradient from the abdomen to the thorax, and vis a tergo (antegrade pressure derived from arterial pressure). 344 Flow is increased by increased fluid intake and particularly by fat ingestion, an effect that may increase flow 10-fold for several hours. Between 60% and 70% of the fat absorbed by the gut passes through the thoracic duct. Chyle contains about 0.5–6 g/dL fat, 3 g/dL of protein, and electrolytes as in serum. There are 400–7000 white cells, mostly T lymphocytes, per milliliter of chyle. 55 Loss of chyle from the body, as occurs with drainage of a chylothorax, has serious consequences, including malnutrition, lymphopenia, and immune compromise. Most of the fat in chyle is carried as triglycerides in the form of chylomicrons, which gives chyle its characteristic milky appearance.
Two other types of pleural fluid may appear milky and should be distinguished: empyema, in which the milkiness is due to leukocytes that, unlike chylomicrons, settle out on standing or centrifugation; and pseudochylous effusions, in which the milkiness is due to cholesterol or lecithin–globulin complexes. Pseudochylous effusions may be distinguished from true chylous effusions by their high cholesterol content and their occurrence in characteristic clinical settings in which there has been longstanding disease with pleural thickening. Not all chylous effusions are milky, and in one series 53% of 38 chylous effusions were initially not diagnosed because they were turbid or bloody. 364 Furthermore, during starvation, as may occur following surgery, flow is reduced and the characteristic milkiness may disappear. 363 The diagnosis of a chylous effusion is made by measuring the triglyceride levels of the effusion. 364 Levels above 110 mg/dL are taken as positive, levels below 50 mg/dL as negative. In patients with intermediate values, lipoprotein electrophoresis365 should be performed. With bilateral effusions it should not be assumed that if one is chylous both are. 91 Chyle is bacteriostatic and does not irritate the pleura, so it does not cause pain, pleuritis, or fibrosis.
Causes of chylothorax
The etiology of chylothoraces can be classified (Box 15.10) as neoplastic, traumatic, idiopathic, or miscellaneous origins.347. and 366. In most series, about 50% have been neoplastic,356.363. and 367. 25% traumatic, and 15% idiopathic. 347
Box 15.10
Neoplastic
• Lymphoma
• Metastatic carcinoma
Traumatic
• Operative
– Cardiac
– Esophageal
– Thoracic
– Cervical
• Penetrating injuries
• Closed injuries
– Major
– Minor
Miscellaneous
• Systemic venous hypertension
• Obstruction of central systemic veins
• Scarring processes
– Mediastinal
– Nodal (filariasis)
• Developmental anomalies
– Thoracic duct atresia
– Lymphangioma
– Lymphangiectasia
– Lymphangioleiomyomatosis and tuberous sclerosis
• Cryptogenic
– Hepatic cirrhosis
– Gorham syndrome
– Mycobacterium tuberculosis
• Sarcoidosis
Neoplastic causes
Trauma
Traumatic chylothorax is most commonly related to surgery, particularly cardiac surgery (Fig. 15.40). It usually arises when the thoracic duct is inadvertently stretched, allowing chyle to seep into surrounding tissues. The frequency of chylothorax with cardiothoracic surgery is between 0.2% and 0.56%,363. and 369. and the condition is most commonly seen with surgery for Fallot tetralogy, patent ductus arteriosus, and coarctation of the aorta. Mobilization of the left subclavian artery appears to be a risk factor. 5 Chylothorax is also described with coronary artery surgery,155.370. and 371. thoracoplasty and pneumonectomy (Fig. 15.42), esophagoscopy, 347 esophageal sclerotherapy, 372 thoracic sympathectomy, and lymph node dissection in the neck or chest.347. and 373. Thoracic esophagectomy carries one of the highest risks for chylothorax, with an incidence of 3% of 320 patients in one series. 374 Some surgeons ligate the thoracic duct at the time of esophagectomy to decrease the incidence of this complication. 5
Closed chest trauma, ranging from major trauma with crush injuries and spinal fractures365. and 375. to very minor injuries, is recognized as causing chylothorax. Chylothorax may also follow penetrating injuries such as stab and bullet wounds to the chest and neck.
Chylothorax may occur following straining during childbirth,128. and 376. vomiting, weight lifting, 347 coughing, 354 and the hyperextension and stretching that accompany yawning. 377 It has been suggested that for such trivial trauma to cause duct rupture, a duct must be distended by a high postprandial lymphatic flow. 344
Idiopathic causes
A considerable number of cases of chylothorax are cryptogenic. This includes most cases of congenital chylothorax. 55 Congenital chylothorax is increasingly diagnosed on antenatal ultrasonography, and is the most common cause of a pleural effusion in the neonatal period. 378 It may be associated with chromosomal anomalies, or with developmental lymphatic anomalies. 356 Neonatal chylothorax may also be related to birth trauma. 356 Most adult cases of cryptogenic chylothorax are thought to be related to minor unnoticed trauma. 55
Miscellaneous
The remaining 10% of chylothoraces have a great variety of causes, many of which fall into one of four subgroups.
• Abnormalities of the lymphatic system. Under this broad heading can be included thoracic duct atresia; 368 tuberous sclerosis and lymphangioleiomyomatosis379 (see Chapter 11, p. 687); pulmonary or mediastinal lymphangiectasia, either per se or as part of Noonan syndrome;348. and 380. and lymphangiomatosis (Fig. 15.43), which may be either localized to the thorax or systemic, often associated with massive osteolysis (Gorham syndrome) (see p. 919).381.382.383.384.385.386.387.388. and 389. In a study by Ryu et al., 379 chylothorax was found in 10% of patients with lymphangiomyomatosis, accounting for 3.5% of cases of chylothorax seen at the Mayo Clinic over a 25-year period. Clusters of lymphangiomyomatous cells may be found in the chylous effusion in this condition. 390
• Impaired venous drainage. Chylothorax has been recorded with raised venous pressure caused by heart disease.367.391. and 392. Thrombosis of the superior vena cava, or of subclavian and brachiocephalic veins is becoming a more common cause of chylothorax (Fig. 15.44).175.176.359.393.394. and 395. Compression of the brachiocephalic vessels by a large multinodular goiter has been reported as a rare cause of chylothorax. 396
Imaging of chylothorax
A chylous effusion on plain radiographs cannot be distinguished from other effusions. Chylothoraces vary from small to massive, can be unilateral or bilateral, and are slightly more frequent on the right side. 405 Reference has already been made to the masslike accumulation in the mediastinum that may precede the effusion406.407. and 408. and the delay, which can vary from days to months, between trauma and the eventual development of chylothorax.362.367. and 409.
As discussed above, the CT attenuation of chyle, despite the fat content, is usually close to water attenuation (Fig. 15.43). Because chyle is non-irritant, chylothorax is usually not associated with pleural thickening or loculation. 55 CT may be helpful in identifying an underlying cause for chylothorax, such as lymphoma, lymphangiomyomatosis, or pulmonary or mediastinal lymphangiomas.
Lymphography was previously commonly performed for chylothorax to directly demonstrate the site of thoracic duct disruption and pleural leakage, identify duct obstruction and collaterals, and demonstrate lymphatic malformations.175.348.397.398.410. and 411. However, it is technically demanding and now rarely performed, as it is unclear that it affects management. 356 Lymphoscintigraphy, with 99mTc sulfur colloid injection into the webs of the feet, may successfully demonstrate thoracic duct obstruction and leakage, though with less anatomic precision than lymphography. 412 The thoracic duct can be opacified by the ingestion of oral ethiodized oil before CT examination, 413 but this is rarely used in practice. Oral ingestion of fatty compounds labeled with iodine-123 has also been used in a few cases to provide scintigraphic localization of thoracic duct anatomy and site of leak.414. and 415.
Pseudochylothorax
Like chylothorax, pseudochylothorax is also a milky effusion, but the milky appearance is the result of cholesterol or lecithin–globulin complexes rather than chylomicrons. 417 Pseudochylothorax characteristically occurs in pleural disease of many years’ duration, with chronic loculated effusion, pleural thickening, and sometimes calcification. It most commonly follows, or is associated with, tuberculosis418 and rheumatoid disease. 419 This clinical context is so characteristic that pseudochylothorax does not usually cause diagnostic confusion with a true chylothorax. Imaging may also help differentiate between these entities, since true chylothorax is not usually associated with pleural thickening, loculation, or calcification.
Hemothorax
Hemothorax usually results from trauma. 420 However, on occasion it occurs in other conditions (Box 15.11). The natural history of hemothorax depends in part on the source of the bleeding. Low-pressure bleeding from the lung tends to stop spontaneously because the pleural fluid compresses and collapses the lung. High-pressure bleeding from systemic vessels is less susceptible to the tamponade effect of pleural fluid, 421 and the bleeding may be rapid and persistent with the formation of a tension hemothorax. 422 In the context of trauma, other causes of rapidly accumulating pleural fluid should be considered, including ruptured esophagus, ruptured thoracic duct, traumatic subarachnoid–pleural fistula, 423 and iatrogenic causes, particularly venous perforation from line placement.
Box 15.11
• Trauma
– Open
– Closed (with or without fracture)
– Iatrogenic
• Infection
– Varicella
• Coagulopathy
– Hemophilia
– Anticoagulants
• Vascular abnormality
– Arteriovenous malformation
– Dissecting aortic aneurysm
– Atherosclerotic aneurysm
• Rib exostosis
• Neurofibromatosis with pregnancy
• Pulmonary and pleural neoplasms
• Extramedullary hematopoiesis
• Pneumothorax
• Catamenial hemothorax (endometriosis)
• Idiopathic
In the acute state nothing on the plain chest radiograph distinguishes hemothorax from other collections of pleural fluid. However, on CT a hemothorax may show areas of hyperdensity (see Fig. 15.14). 424 With clotting of the blood, loculation tends to occur and masslike fibrin bodies may form.57. and 425. Hemothorax may eventually organize and cause massive pleural thickening (fibrothorax), necessitating decortication, a complication that can be avoided by early evacuation of the pleural space.
PLEURAL THICKENING
Pleural thickening can be localized or diffuse, and usually represents the organized end stage of a variety of active processes, particularly infective and noninfective inflammation, hemothorax, and asbestos- and drug-related disease (see Box 15.12). It is virtually always present after thoracotomy and pleurodesis. 426 The most common causes are probably hemothorax, bacterial infection, and tuberculosis. 55 Particularly marked pleural thickening is seen following tuberculosis or irradiation. Identification of diffuse pleural thickening is important because it is commonly associated with substantial restrictive physiologic impairment. It can be important to try to distinguish between diffuse pleural thickening, which may be due to a large variety of causes, and localized pleural plaques which are usually related to asbestos exposure (see Chapter 8, p. 472). The presence of bilateral abnormality favors asbestos-related disease. 7 Diffuse postinflammatory pleural thickening usually involves the more dependent lateral and posterior parts of the chest, and almost always involves the costophrenic sulci (Fig. 15.45), while pleural plaques present with localized areas of soft tissue density along the chest wall. There may be ipsilateral rib enlargement or periosteal thickening, particularly with chronic pleural infection such as tuberculosis. 427
Box 15.12
• Infection
– Tuberculosis
– Empyema
• Hemothorax
• Asbestos exposure
• Surgery or pleurodesis
• Radiation
• Malignancy
– Metastasis
– Mesothelioma
– Leukemia
– Lymphoma
En face, extensive pleural thickening gives a veil-like opacity that has no clear margins and crosses known pulmonary boundaries. Tangentially, it appears as a soft tissue density immediately inside and parallel to the chest wall, sharply marginated on its inner aspect and fading into the soft tissues of the chest wall laterally. Such pleural thickening can extend into and thicken fissures. Linear parenchymal opacities or rounded atelectasis are commonly seen.
Pleural thickening can usually be distinguished from pleural fluid by careful radiographic analysis. The costophrenic angle blunting caused by pleural thickening is often angular, distinguishing it from the more smoothly curvilinear margin of pleural fluid, and often spares either the posterior or lateral costophrenic sulcus. In cases of uncertainty, decubitus radiographs or ultrasonography can be helpful. On ultrasonography, pleural thickening produces a homogeneously echo-dense layer subjacent to the chest wall, but it cannot be reliably detected unless it is about 1 cm or more thick. 84 There is no posterior echo enhancement, but this is often difficult to assess because the soft tissue–lung interface is normally so reflective.
On CT, pleural thickening is detected as a layer of soft tissue opacity lying at the chest wall–lung interface. Pleural thickening is best assessed inside ribs where there should be no discernible soft tissue; exceptions to this ‘rule’ are discussed on pages 53 and 54 (Chapter 2). 428 Paravertebrally any thickening of the normally insignificant pleural line is abnormal. Thin-section CT is very sensitive and can detect thickening of the order of 1–2 mm. The extrapleural fat layer, which is normally absent or relatively thin, thickens in chronic pleural disease, particularly with chronic empyema,428.429.430. and 431. making appreciation of pleural thickening easier. When this fat has higher density than usual, it suggests that there is active inflammation in the pleural space.93.94. and 432. Both the distribution and morphology87 of diffuse pleural thickening are helpful in identifying a cause. In one study the specificity of various CT signs in differentiating a malignant from a benign pleural process was measured. The four most useful signs of malignancy (with specificities) were circumferential thickening (100%), nodularity (94%), parietal thickening greater than 1 cm (94%), and mediastinal pleural involvement. 433 Positron emission tomography (PET) with [18 F]-2-fluoro-2-deoxy-d-glucose (FDG) can have a role in the differentiation between malignant and benign pleural disease. 434 Malignant effusions may be associated with increased metabolic activity either in the pleura or in the pleural fluid. In a study of 23 patients, FDG-PET correctly identified all 16 cases of malignant pleural infiltration; there was intense uptake of FDG in 14 out of 16 cases (Fig. 15.46). 435 Seven out of nine benign lesions were characterized by an absence of tracer uptake but there was moderate uptake in two patients, one with a parapneumonic effusion and one with tuberculous pleurisy. 435 Another study found that MRI was more accurate than CT in diagnosis of malignant pleural disease: malignant disease was associated with increased signal intensity on T2-weighted images, and with enhancement following gadolinium administration. 436
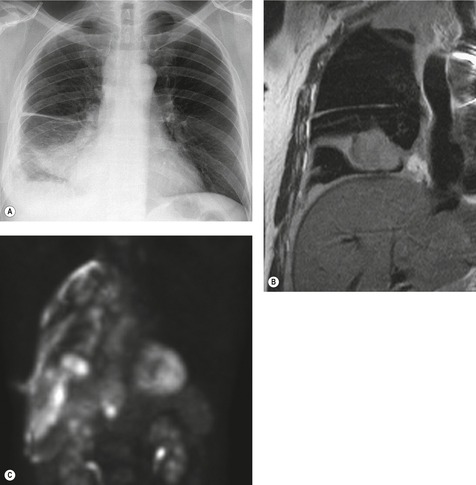 |
| Fig. 15.46 Activity of PET in malignant pleural disease due to mesothelioma (same patient as Fig 15.32). A, Chest radiograph shows circumferential pleural thickening. B, Coronal MR image confirms the presence of circumferential pleural thickening extending into the costophrenic sulci, with a mass in the major fissure. C, Coronal PET image obtained with FDG shows circumferential increase in metabolic activity, extending into the fissures. |
Pleural thickening, particularly when generalized and not associated with effusion, is often correctly dismissed as an inactive residuum. Care, however, must be taken to distinguish it from various active processes, some of which are neoplastic. Although a number of these conditions tend to give plaquelike, nodular, or irregular shadowing (Fig. 15.46), they occasionally closely resemble simple inactive pleural thickening. Disorders to consider include mycetoma-related pleural thickening, 437 mesothelioma, diffuse pleural metastases (e.g. from thymoma438 or breast cancer), leukemia, 439 sarcoidosis, lymphoma, and Wegener granulomatosis. It should also be remembered that a thick pleural peel occurring following empyema will usually decrease progressively over the subsequent 12 weeks. 440
There are several uncommon syndromes of idiopathic bilateral pleural thickening. In the entity of cryptogenic bilateral fibrosing pleuritis there is widespread thickening of the pleura preceded by effusions (Fig. 15.47).441. and 442. Patients may have an elevated erythrocyte sedimentation rate, restrictive lung function, and evidence of rounded atelectasis on CT. 441 Another uncommon idiopathic syndrome is idiopathic pleuroparenchymal fibroelastosis, associated with progressive apical pleural and subpleural fibrosis, and restrictive physiology, further discussed on page 584 (Chapter 10).443. and 444.
Mimics of pleural thickening
Pleural thickening must be differentiated from apical pleural caps and extrapleural fat. The apical pleural cap, though it looks like pleural thickening, is usually due either to extrapleural fat or to subpleural fibrosis. Older publications refer to serratus anterior shadows and rib companion shadows as mimics of pleural plaques on chest radiographs.445. and 446. These are not discussed here because it is very rare for the serratus anterior muscle or ribs to be so sharply outlined by air as to produce a radiographically distinct interface.
Extrapleural fat
Extrapleural fat may generate confusing shadows that can resemble generalized pleural thickening or plaques (Fig. 15.48).447. and 448. The distribution varies from patient to patient. Sometimes the fat is widely distributed, mimicking a pleural peel. At other times, it is localized and develops particularly over the fourth to eighth ribs between the anterior axillary line and the rib angles. 447 Excess thoracic fat is more common in obese patients, and associated with mediastinal lipomatosis, but may also be found in thinner individuals. Extrapleural fat may be distinguished from pleural thickening by its symmetry and its undulating outline, often extending to the lung apices but sparing the costophrenic sulci. 447 If doubt remains, CT will readily differentiate thickening from fat, but this distinction is not usually clinically important. 447 Extrapleural fat is further discussed on page 473.
Apical pleural cap
An idiopathic apical pleural cap is an irregular, usually homogeneous, soft tissue density that may be found at the extreme lung apex (Fig. 15.49). 449 The lower border is usually sharply marginated and may be smoothly curvilinear, tented, or undulating. 450 Caps are usually less than 5 mm thick, but the width is variable. In two series, caps were about as common unilaterally (11% and 7%) as bilaterally (11% and 12%).450. and 451. When bilateral the caps were usually asymmetric. The frequency of occurrence increases with age: 6.2% up to 45 years of age and 15.9% over 45 years of age. 449 The opacity is formed by an apical subpleural scar that is nonspecific and unrelated to tuberculosis. 452
In contrast to the smooth or undulating outline of the apical cap on the chest radiograph, CT usually shows a subpleural irregular linear abnormality, consistent with the pathologic findings of dense subpleural fibrosis, and sometimes difficult to distinguish from a spiculated lung cancer (Fig. 15.50). Indeed, Yousem453 presented a series of 13 such cases which had been resected because of suspected lung cancer. Unlike most lung cancers, these subpleural scars are usually much smaller in craniocaudal dimension than in axial dimensions. Other entities to consider in the differential diagnosis of an idiopathic apical cap include tuberculosis, fungal infection, mycetoma, radiation pleuritis, lymphoma, pleural and extrapleural neoplasms, extrapleural hematoma, prominent subclavian artery, 454 and, most commonly, extrapleural fat. 455 A CT study has shown somewhat surprisingly that the bulk of the apical ‘pleural’ opacity associated with previous tuberculosis is caused by a greatly thickened (up to 25 mm) layer of extrapleural fat. 456 The most important differential diagnosis is with Pancoast tumor, which should be suspected if there is marked asymmetry or nodularity of apical pleural thickening, if the patient has local pain, and particularly if there is underlying bone destruction (Fig. 15.51) (see Chapter 13, p. 805). 454
RADIOGRAPHIC APPEARANCE FOLLOWING PLEURODESIS
The most common indications for pleurodesis are recurrent pneumothorax, and malignant pleural effusion. The perfect agent for pleurodesis has not yet been found, but talc is increasingly used in place of chemical agents such as tetracycline or bleomycin, because of its higher success rates and lower rate of local and systemic symptoms.329.457. and 458. Talc may be administered either as an aerosolized powder at thoracoscopy,459. and 460. or as a slurry via large-bore or small-bore chest tubes at the bedside.329.461. and 462.
After pleurodesis, the pleural space usually undergoes a phase of organization, with pleural thickening and loculations evident on chest radiograph; about 60% of patients have radiographically visible pleural thickening at long-term follow-up. 426 On CT scans obtained after talc pleurodesis, the pleural space usually reveals variable degrees of pleural thickening and nodularity, often with a residual loculated effusion. High-attenuation areas representing talc deposits are usually seen, and may mimic pleural calcification (Fig. 15.52). 463 These areas are usually in the posterior basal pleura, may extend into the fissures, and may be seen along both the parietal and visceral pleura if there is a residual effusion. 464 Inflammation related to talc pleurodesis commonly causes focal or diffusely increased metabolic activity on FDG-PET scans, with standardized uptake values up to 16.3, simulating malignancy: identification of associated high-attenuation areas on CT can be pivotal in confirming the correct diagnosis.465. and 466. The increased activity may persist for years after pleurodesis. The use of talc as a pleurodesis agent remains controversial, mainly because of recurrent reports of acute respiratory distress syndrome (ARDS) or lung edema occurring after talc pleurodesis.459.




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree