MEDIASTINAL DISEASES
Imaging techniques
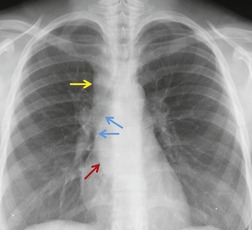
The chest radiograph is usually the first imaging study obtained in a patient with a known or suspected mediastinal or hilar mass. Furthermore, mediastinal or hilar abnormalities are often discovered serendipitously on chest radiographs obtained for other purposes. Thus, the role of chest radiography for detection of hilar and mediastinal abnormalities remains essential, and thorough knowledge of the relevant radiographic anatomy is of utmost importance to the practicing radiologist.
Despite the advent of cross-sectional imaging techniques such as computed tomography (CT) or magnetic resonance imaging (MRI), the chest radiograph remains important for localization of the mass (useful for formulating an appropriate differential diagnosis) and, in some instances, for characterization of the lesion. Some abnormalities, such as vascular lesions or mediastinal lipomatosis, may have a sufficiently characteristic appearance on the chest radiograph to obviate further evaluation. Findings of calcification within the mass on chest radiography can also be a clue to the correct diagnosis. However, in most cases, once a mediastinal or hilar abnormality is detected, or at least suspected, on the chest radiograph, cross-sectional imaging is performed. CT or MRI are used to assess the location and extent of the lesion and, because of their superior contrast resolution, are also used to characterize the tissue components of the mass. CT or MRI are also quite useful for distinguishing vascular lesions or benign processes of the mediastinum such as lipomatosis from pathologic conditions that warrant further investigation.
Ultrasonography can also be useful for imaging mediastinal abnormalities in selected patients. Because it does not use ionizing radiation, ultrasonography may be preferred to CT for evaluation of some mediastinal masses in children, such as mediastinal cysts. 1 If the lesion is thought to arise from the heart or great vessels, either transthoracic or endoscopic ultrasonography may be the first line of investigation. Furthermore, ultrasonography can be useful for guiding biopsy of mediastinal masses. 2
Although cross-sectional imaging is primarily used to evaluate abnormalities detected by radiography, it may also be performed in certain situations when the chest radiograph is normal. For example, CT may be performed in patients with myasthenia gravis even if the chest radiograph is normal, because of the association between myasthenia gravis and thymoma. Furthermore, malignancies such as lung cancer have a predilection to metastasize to mediastinal lymph nodes. These metastases may not be visible on the chest radiograph and CT is used to further assess the mediastinal nodes in such patients.
CT is now the mainstay for the evaluation of known or suspected mediastinal or hilar abnormalities. However, MRI is sometimes used to further evaluate the location and extent of mediastinal or hilar disease because of its high contrast resolution compared with CT and lack of ionizing radiation. Further, MRI may be the method of choice for imaging suspected neurogenic tumors because it not only shows the size, location, and internal features of the lesions, but because it more clearly depicts spinal involvement. 3 MRI is also useful for confirming the cystic nature of mediastinal lesions that appear solid on CT, such as bronchogenic cysts, and for demonstrating vascular structures in patients for whom administration of iodinated intravenous contrast is contraindicated. 3 Two potential disadvantages of MRI compared with CT are its poor depiction of calcification and comparatively poorer spatial resolution.
Multidetector spiral CT has further improved the ability of CT to image the mediastinum. 3 By markedly shortening scan time, respiratory motion artifacts are limited and the dose of iodinated contrast can be reduced. 4 Multidetector CT datasets can also be effectively reconstructed in a variety of nonaxial planes, often facilitating interpretation of mediastinal abnormalities. The application of nonaxial two- and three-dimensional reconstruction techniques has proved most useful for imaging abnormalities of the central airways and great vessels. 5 By presenting anatomic information in a context more familiar to referring clinicians, these reconstructed images may show the location and extent of an abnormality in a way that imaging reports and axial CT images do not.
Positron emission tomography (PET) is a physiologic imaging technique that uses metabolic markers labeled with positron-emitting radionuclides such as fluorine-18, carbon-11, or oxygen-15. 6 [18F]2-fluoro-2-deoxy-d-glucose (FDG), a d-glucose analog labeled with fluorine-18, is ideally suited for tumor imaging. 7 PET performed with this agent (FDG-PET) exploits the differences in glucose metabolism between normal and neoplastic cells. After intravenous administration, FDG preferentially accumulates in neoplastic cells allowing accurate, noninvasive differentiation of benign from malignant abnormalities by PET imaging. 8 FDG-PET imaging has proved quite useful for staging patients with a variety of systemic malignancies that affect the mediastinum, including lymphoma and lung cancer, and has become a mainstay for evaluation of such lesions.9. and 10. FDG-PET imaging can also play an important role in staging patients with primary mediastinal malignancies such as thymic epithelial neoplasm and nonseminomatous germ cell tumors.11.12. and 13. However, FDG-PET has had a more limited role in evaluation of localized mediastinal processes such as neurogenic tumors. In these tumors, accurate information about the location and anatomic extent of disease, as provided by MRI or CT, is likely of greater importance than the assessment of metabolic activity of the tumor.
Frequency of mediastinal masses
The relative frequency of various mediastinal lesions is difficult to ascertain because most published series are biased toward patients whose lesions undergo biopsy or resection. Some common mediastinal masses such as thyroid goiter, aortic aneurysms, or lymphadenopathy in patients with previously established diagnoses such as lymphoma or sarcoidosis are underrepresented in many surgical reviews. The relative frequency of lesions in several large series is shown in Table 14.1. In the Mayo Clinic series, 14 about 75% of mediastinal masses in both adults and children were benign and completely resectable and 25% were malignant. The authors14 highlighted the significant differences in the relative frequencies of mediastinal lesions in children and adults. Neurogenic tumors, germ cell neoplasms, and foregut cysts accounted for almost 80% of the masses seen in children. Conversely, primary thymic neoplasms, pericardial cysts, and thoracic goiters were rare in childhood. 14 Another retrospective series, however, found that the only significant differences between the adult and pediatric populations were a higher frequency of lymphoma in adults and of neurogenic tumors in children. 15 Surprisingly, the frequency of thymic tumors in adults and children was not significantly different in that series. 15 Temes et al. 16 and Takeda et al., 17 however, reported a significantly lower frequency of thymic tumors and a higher frequency of neurogenic tumors in children. Although all of these series were limited by the biases inherent in retrospective, single-institution, surgical studies, a few trends emerge:18.19. and 20.
• Neurogenic tumors are more frequent in children than in adults, perhaps reflecting the prevalence of neuroblastoma in that population.
• Thymic and thyroid tumors are more common in adults.
• Lymphoma tends to occur as a mediastinal mass with roughly equal frequency in adults and children, as do benign cysts of the mediastinum.
| *Only mediastinal malignancies were included. | ||||||||||
| †Not reported as a separate category. | ||||||||||
| Series | Wychulis et al. 14 | Benjamin et al. 18 | Cohen et al. 19 | Azarow et al. 15 | Whooley et al. 20 | Temes et al. 16* | Takeda et al. 17 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Population | All | All | All | Pediatric | Adult | All | Adult | Pediatric | Adult | Pediatric |
| Number | 1064 | 214 | 230 | 62 | 195 | 124 | 197 | 22 | 676 | 130 |
| Neurogenic | 20 | 23 | 17 | 32 | 12 | 12 | 1 | 23 | 11 | 46 |
| Thymic | 19 | 21 | 24 | 33 | 26 | 33 | 16 | 0 | 36 | 4 |
| Lymphoma | 10 | 15 | 16 | 6 | 21 | 19 | 55 | 55 | 12 | 13 |
| Germ cell | 9 | 13 | 10 | 6 | 12 | 23 | 15 | 18 | 16 | 19 |
| Benign cyst | 18 | 7 | 20 | 23 | 16 | 4 | NA | NA | 14 | 10 |
| Thyroid | 5 | 11 | 2 | 0 | † | 0 | NA | NA | 4 | † |
| Granuloma | 6 | † | 0 | 0 | † | 0 | NA | NA | † | † |
| Mesenchymal | 6 | 3 | 4 | 0 | † | 5 | 6 | 4 | † | † |
| Primary carcinoma | 2 | † | † | 0 | † | 0 | 0 | 0 | † | † |
| Vascular tumor/malformation | † | 7 | 2 | 0 | † | 0 | NA | NA | † | † |
| Miscellaneous | 5 | † | 5 | 0 | 13 | 4 | 7 | 0 | 7 | 8 |
Differential diagnosis of mediastinal masses
Mediastinal masses are classically defined and discussed according to their location in the anterior, middle, or posterior mediastinal compartments. This classification is primarily a matter of descriptive convenience because there are no anatomic boundaries that limit growth between these various compartments. Indeed, many radiologists do not use these terms in the manner defined by anatomy textbooks. As Heitzman21 noted, apart from being useful for remembering that thymic lesions, thyroid masses, and germ cell tumors are found in the anterior mediastinum and that most neurogenic tumors are posteriorly situated, this simple classification ‘tends to constrict thinking and minimizes more detailed anatomic analysis’. Much more important is the accurate assessment of the location of the mass, together with a description of its size, shape, and characteristics such as CT attenuation, magnetic resonance (MR) signal intensity, or metabolic activity on PET. Cross-sectional imaging techniques, notably CT, provide the best information with which to refine the differential diagnosis and, on occasion, suggest a specific diagnosis.22. and 23.
The differential diagnosis of a mediastinal mass depends on the age of the patient, the location of the mass, the imaging technique used to evaluate the mass, and findings on that imaging examination. For example, Ahn et al. 24 analyzed chest radiographs and CT of 128 patients with anterior mediastinal masses and showed that, using the chest radiograph, the first-choice diagnosis was correct in 36% of cases; using CT, the first-choice diagnosis was correct in 48%. Using chest radiographs, the correct diagnosis was included among the top three choices in 59% of cases; using CT, the correct diagnosis was included among the top three choices in 73% of cases. This serves to emphasize that CT can not only help narrow the differential diagnosis, but may also reflect the rather limited range of pathologies encountered in the anterior mediastinum.
The first step in the differential diagnosis of a mediastinal mass is to be sure that the mass arises from the mediastinum rather than from contiguous lung, pleura, spine or sternum. Masses that lie deep to mediastinal vessels are certainly mediastinal in origin and masses that arise from the sternum or spine should be obvious at CT. The interface with the adjacent lung is a most useful sign, particularly at CT. With few exceptions, a mass with spiculated, nodular, or irregular borders arises in the lung; likewise, a well-marginated mass with a broad base against the mediastinum arises either from the mediastinum or from the mediastinal pleura. 25 Masses arising from the mediastinal pleura project into the lung and usually have obtuse rather than acute angles at their margins.
Some general comments regarding patient age, CT attenuation, or MR signal intensity and multiplicity are made here, since all three features are relevant, whatever the location of the mass:
• Lymphoma, benign thymic enlargement, germ cell tumors, foregut cysts and neurogenic tumors of ganglion cell origin make up 80% of mediastinal masses in children. 26 In adults, lymphoma, metastatic carcinoma to lymph nodes, intrathoracic goiter, thymoma, neurogenic tumors of nerve sheath origin, aortic aneurysms, germ cell tumors, and foregut cysts are the prime considerations.
• Lesions that are of higher attenuation than muscle on noncontrast CT scans are usually calcified, have high iodine content (indicating thyroid tissue), or contain areas of acute hemorrhage. 27 Furthermore:
• Irregular, granular, or eggshell calcification within multiple small mediastinal masses limits the differential diagnosis, for practical purposes, to lymphadenopathy due to such benign conditions as granulomatous infection, coal worker pneumoconiosis, silicosis, and sarcoidosis. Amyloidosis, treated lymphoma, metastasis, and Castleman disease may be an occasional cause.
• Calcification in a solitary mass has a broader differential diagnosis. Neurogenic tumors may calcify, as may thymoma and germ cell tumors.
• Curvilinear calcification is seen in the walls of foregut cysts, mature teratoma, and, occasionally, pericardial cysts. Untreated lymphoma almost never calcifies. Aneurysms of the aorta or its major branches frequently have curvilinear calcification in their walls or in thrombus lining the aneurysm. This pattern of calcification, along with the observation that the mass arises from, or is in intimate contact with, the aorta or branch vessels, suggests the correct diagnosis.
• Lesions that are of homogeneous water attenuation on CT or have characteristics of water on MRI, and have a thin wall of uniform thickness are most likely congenital cysts, pericardial recesses, meningoceles, or lymphangiomas. Necrotic malignant or benign neoplasms are usually heterogeneous and have thick or irregular walls. 28 Some neurogenic tumors may be of low attenuation on CT; however, they are typically of higher attenuation than water, occur in characteristic locations, and enhance after administration of contrast material.
• Lesions that contain fat on CT or MRI include collections of normal fat (epicardial fat pads, lipomatosis, and herniated abdominal fat), lipomas, lipoblastomas, liposarcomas, extramedullary hematopoiesis, teratomas, thymolipoma, and fat-replaced lymph nodes. 29 A fat–fluid level within a cystic mass is pathognomonic of mature teratoma. Benign lipomas and thymolipomas are composed almost entirely of fat and should contain but a few thin strands of soft tissue. Liposarcomas are rare and usually manifest as mixed fat and soft tissue masses.
• Contrast enhancement, either by iodinated contrast material at CT or by gadolinium-based contrast material at MRI, is an important feature and can be diagnostic of a vascular lesion such as an aneurysm. A minor degree of enhancement of the soft tissue component of a mass is a nonspecific finding. However, marked enhancement suggests thyroid tissue, vascular tumors such as paragangliomas, or Castleman disease. 30
• The finding of multiple small masses within the mediastinum is suggestive of lymphadenopathy. The nodes may be separated by fat or may conglomerate into multiple larger lobulated masses.
• At MRI, most mediastinal masses are of low-to-intermediate signal on T1-weighted images and relatively high signal on T2-weighted images.
• Those that contain water, or fluid similar to water, have uniformly low signal on T1-weighted images and uniformly very high signal on T2-weighted or short tau inversion recovery (STIR) images.
• Lesions that contain fat or subacute hemorrhage have substantially higher signal intensity than muscle on T1-weighted images. 31 The differential diagnosis of masses with areas of high signal intensity on T1-weighted images is extensive, because many primary and secondary mediastinal tumors occasionally contain such foci even in the absence of fat or recent hemorrhage.
• Furthermore, cysts that contain proteinaceous debris may be of high signal intensity on T1-weighted MRI. Thus, mediastinal masses that may have signal intensity similar to or near that of fat on T1-weighted images include neurogenic tumors, lipomas, teratomas, foregut cysts, lymphangioma, paraganglioma, carcinoid tumors, and a variety of primary and secondary carcinomas.31. and 32.
Finally, location is clearly of great importance for differentiating mediastinal masses. Cross-sectional imaging, particularly CT, is the mainstay for evaluation of known or suspected mediastinal masses. Thus, the differential diagnosis is best discussed by location on cross-sectional imaging.
Prevascular masses
Prevascular masses (Box 14.1) are located anterior to the ascending aorta and branch vessels. Almost all masses in this location33 are thyroid or thymic masses, germ cell tumors, or lymphadenopathy. Thyroid masses can usually be specifically diagnosed or excluded based on their contiguity with the thyroid gland in the neck and their high CT attenuation on both pre- and postcontrast scans. In addition, most thyroid lesions are heterogeneous and have focal cysts as well as one or more areas of discrete calcification. A mass located superiorly in the anterior mediastinum that causes lateral deviation of the trachea is likely to be of thyroid origin.
Box 14.1
Common
• Thyroid masses
• Thymic lesions
• Germ cell tumors
• Lymphadenopathy
Uncommon
• Parathyroid adenoma
• Lymphangioma (cystic hygroma)
• Pericardial cyst
• Aortic body paraganglioma
• Mesenchymal tumor
• Aneurysm
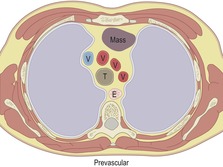 |
T, trachea; E, esophagus; V, great vessel.
Thymic masses and germ cell tumors can have a similar appearance on cross-sectional imaging. Clinical and laboratory features may help distinguish them. For example, myasthenia gravis, red cell aplasia, and hypogammaglobulinemia are associated with thymoma, whereas high α-fetoprotein (AFP) or human chorionic gonadotropin (hCG) levels are associated with malignant germ cell tumors. If there is an associated pleural mass, then lymphoma or intrapleural spread of thymoma becomes a strong possibility. If fat, cartilaginous calcification, or teeth are present in the mass, mature teratoma is the most likely diagnosis.
More unusual causes of masses anterior to the aorta and branch vessels are parathyroid adenoma, lymphangioma (cystic hygroma), pericardial cyst, aortic body paraganglioma, lipoma, liposarcoma or other mesenchymal tumors, or aneurysms. Many of these masses have features that permit a specific diagnosis to be made. Parathyroid adenomas are usually associated with clinical features of hyperparathyroidism and are discovered in the quest for an ectopic parathyroid gland. Lymphangiomas, because they are composed largely of lymph-filled spaces, show numerous cysts on CT. Cystic hygroma should be a serious consideration for a prevascular mass that extends into the neck in a child. Lipomas may be indistinguishable from normal fat collections but are readily distinguished from more significant fat-containing mediastinal masses. Liposarcomas show a mixture of fat and irregular strands or masses of soft tissue. Pericardial cysts are, in general, of uniform water density with a thin, uniform thickness wall, and they need only be considered when the mass in question is in contact with the pericardium. It should be remembered, however, that the pericardium extends to the level of the junction between the proximal and middle thirds of the ascending aorta. Mesenchymal tumors such as sarcomas often have no distinguishing features.
Paracardiac masses
The likely diagnoses for paracardiac masses (Box 14.2) that contact the diaphragm are pericardial cyst, diaphragmatic hernia, fat pad, lymphadenopathy, or, in patients with portal hypertension, cardiophrenic varices.34. and 35. Most pericardial cysts are diagnosable by their uniform water attenuation on CT and their thin walls. Morgagni hernias are recognized by the omental fat within the hernia and sometimes by opacified bowel either within the mass or leading into it. If the mass is not in contact with the diaphragm, the differential diagnosis broadens to include germ cell tumors, mesenchymal and pericardial tumors, and thymic masses. Approximately 20% of thymomas are found in a paracardiac location, though contact with the diaphragm is very unusual. Lack of contact with the diaphragm excludes diaphragmatic hernia.
Box 14.2
Common
• Pericardial cyst
• Morgagni hernia
• Epicardial (anterior) fat pad
• Lymphadenopathy
Uncommon
• Thymoma
• Germ cell tumor
• Mesenchymal tumor
• Varices
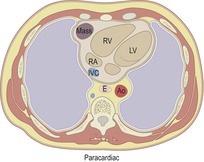 |
RV, right ventricle; LV, left ventricle; RA, right atrium; IVC, inferior vena cava; E, esophagus; Ao, aorta.
Paratracheal, subcarinal, and paraesophageal masses
Paratracheal, subcarinal, and paraesophageal masses (Box 14.3) are considered together because the trachea, central bronchi, and esophagus are contained within a common fascial sheath. This compartment continues into the neck around the airway, the esophagus, and pharynx. Prime considerations for nonvascular masses in these locations are lymphadenopathy, intrathoracic thyroid mass, foregut cysts, esophageal tumors, hiatal hernias, and paraspinal masses encroaching on the middle mediastinum. In terms of frequency, lymphadenopathy is by far the most frequent. Masses deep to the azygos vein in either the right paratracheal area or in the pretracheal or precarinal space are almost invariably enlarged lymph nodes. For masses arising in the aortopulmonary window, the only other alternative is aortic aneurysm – a diagnosis that can be readily confirmed or excluded with contrast-enhanced CT or MRI. As mentioned earlier, lymphadenopathy is frequently multifocal and, in the case of metastatic carcinoma, the primary tumor is usually already known. Bronchogenic cyst can be diagnosed with confidence if the criteria of a simple cyst are met. But many bronchogenic cysts do not meet these criteria and these, therefore, are included in the differential diagnosis of a solid mediastinal mass. 36 Thyroid masses that pass lateral to or posterior to the trachea are distinctive, partly because of the signs discussed below, but also because thyroid masses show far greater contact, displacement, and compression of the trachea than do lymph nodes. Separation of the trachea from the esophagus is a characteristic shared only by thyroid masses, bronchogenic cysts, esophageal tumors, and an aberrant origin of the left pulmonary artery. Aortic arch anomalies, though they deform the trachea and esophagus in various ways, do not pass between these two structures.
Box 14.3
• Lymphadenopathy
• Foregut malformations/cysts
• Esophageal tumors
• Thyroid lesions
• Hiatal hernia
• Aneurysms
• Vascular anomalies
• Pancreatic pseudocyst
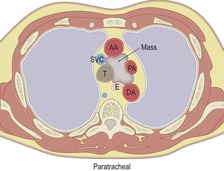 |
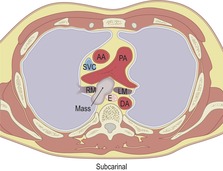 |
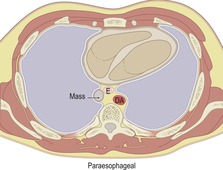 |
Ao, aorta; SVC, superior vena cava; AA, ascending aorta; PA, pulmonary artery; T, trachea; E, esophagus; DA, descending aorta; RM, right main bronchus; LM, left main bronchus; RV, right ventricle; LV, left ventricle.
Esophageal tumors very rarely present as unexpected mediastinal masses. Patients with esophageal carcinoma, the most common esophageal tumor, nearly always present with dysphagia at a time when the tumor mass is relatively small. Although the tumor can sometimes be seen as a mass on plain chest radiographs and may be recognized at CT, the diagnosis of esophageal carcinoma is usually made at endoscopy or barium swallow examination. Leiomyoma or other mesenchymal tumors of the esophagus may grow to a considerable size without causing dysphagia and may, on occasion, present as a mediastinal mass on chest radiography or CT. Hiatal hernia is an exceedingly common cause of a mediastinal mass in the region of the lower esophagus. The diagnosis from chest radiographs is so straightforward and reliable that barium swallow or CT should rarely be required to confirm the diagnosis.
As discussed below, a number of vascular anomalies may mimic a paratracheal mass on chest radiographs and sometimes even on CT.
Paravertebral masses
Strictly speaking, masses situated on either side of the vertebral column (Box 14.4) are outside the mediastinum since, according to anatomists’ definitions, the mediastinum lies anterior to the spine. However, it is standard practice among radiologists and thoracic surgeons to label paraspinal masses as posterior mediastinal masses.
Box 14.4
Common
• Neurogenic tumors
• Lymphadenopathy
Uncommon
• Extramedullary hematopoiesis
• Pancreatic pseudocyst
• Mesenchymal tumors
• Esophageal lesions
• Paraspinal abscess or hematoma
• Aneurysm
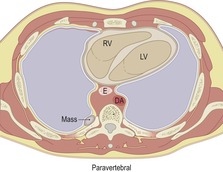 |
DA, descending aorta; E, esophagus; RV, right ventricle; LV, left ventricle.
Neurogenic lesions and lymphadenopathy dominate the differential diagnosis for paraspinal masses. Lymphadenopathy is rarely confined to the paraspinal areas; usually it is accompanied by enlarged lymph nodes in adjacent mediastinal or retroperitoneal areas. The most common causes of posterior mediastinal lymph node enlargement are lymphoma and metastatic carcinoma from genitourinary primary tumors. Other, less common, causes of paraspinal masses include metastases from other sites, extramedullary hematopoiesis, pancreatic pseudocyst, mesenchymal tumors such as lipoma, fibroma, chordoma, and hemangioma, and lesions arising from the esophagus, pharynx, spine, or aorta. The esophageal or pharyngeal lesions that may project posteriorly include leiomyoma, foregut cyst, and congenital or acquired diverticula of the esophagus. The spinal origin of lesions such as paraspinal abscess, tumors of the vertebral body that have spread into the adjacent paravertebral space, or hematomas from trauma to the spine, are usually readily diagnosed by observing corresponding changes in the spine.
Aneurysms of the descending aorta that truly mimic mediastinal masses are uncommon. Most large aneurysms in this location are usually obvious. Saccular aneurysms that could be confused with a mass show a broad base on the aorta and almost always have curvilinear calcification in their walls. The diagnosis is readily made on CT or MRI when opacification of the lumen can be demonstrated.
SPECIFIC MEDIASTINAL LESIONS
Cysts or cystlike lesions
True mediastinal cysts or cystlike lesions (Box 14.5) are usually developmental in origin and include bronchogenic cysts, esophageal duplication cysts, neurenteric cysts, and pericardial cysts. In one series of 105 patients with mediastinal cysts, 45% were bronchogenic, 28% were thymic, and 11% were pericardial cysts. 37 The remainder were esophageal duplication cysts, meningoceles, or thoracic duct cysts. Bronchogenic cysts are discussed in Chapter 16 (p 1089). Parathyroid cysts, thymic cysts, and lymphangioma are discussed on pages 938, 955 and 1087, respectively.
Box 14.5
• Foregut duplication cysts
– Bronchogenic cysts
– Esophageal duplication cysts
– Neurenteric cysts
• Pericardial cysts
• Thymic cysts
• Parathyroid cysts
• Pancreatic pseudocysts
• Lymphangiomas (cystic hygromas)
• Lymphoceles
• Thoracic duct cysts
• Meningoceles
Distinguishing between the various cysts and cystlike lesions of the mediastinum is not always straightforward. For example, a cyst deep in the wall in the esophagus, and unquestionably by all anatomic criteria an esophageal duplication cyst, may contain respiratory epithelium. In order to emphasize their origin from the embryological foregut, bronchogenic, esophageal, and neurenteric cysts are often collectively referred to as foregut duplication cysts.38. and 39. Foregut duplication cysts account for approximately 20% of all mediastinal masses.37.40. and 41. Bronchogenic cysts are the most common mediastinal foregut cysts; esophageal duplication and neurenteric cysts are less common. Mediastinal cysts that contain cartilage are classified as bronchogenic cysts and those that contain gastric epithelium are classified as enteric duplication cysts. Those cysts with seromucinous glands are considered as probably, although not definitely, respiratory in origin. Most congenital mediastinal cysts are lined by respiratory epithelium and these are usually labeled bronchogenic cysts even though their precise origin can only be conjectured. 39
Esophageal duplication cysts
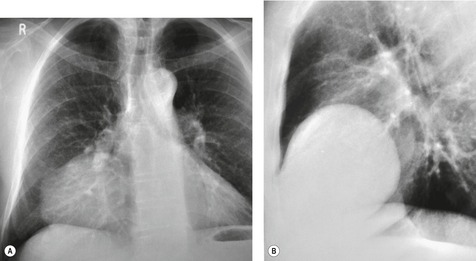
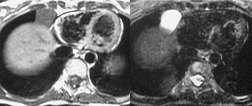
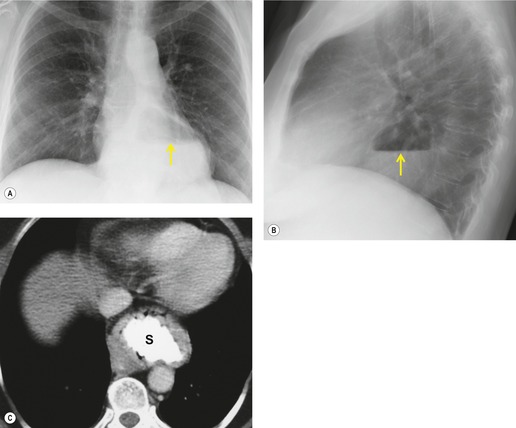
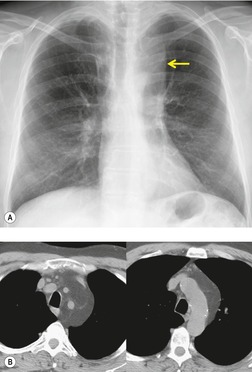
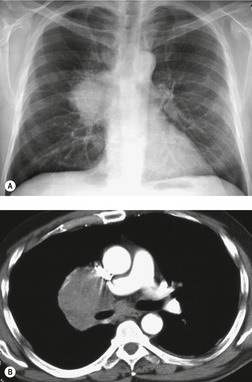
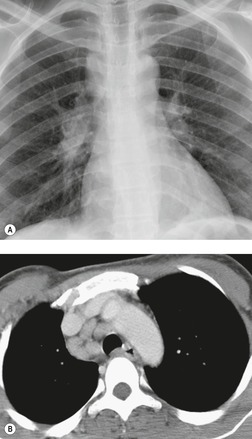
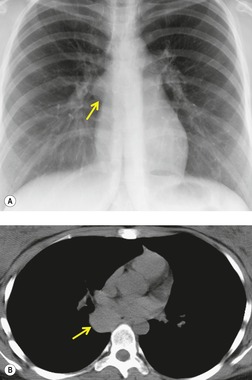
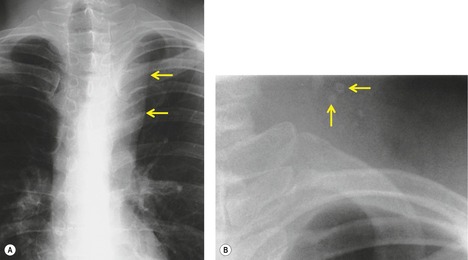
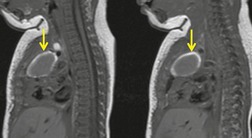
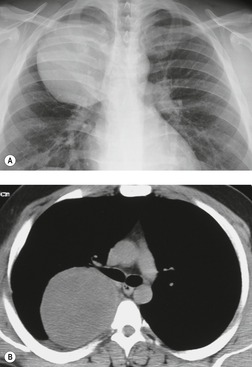
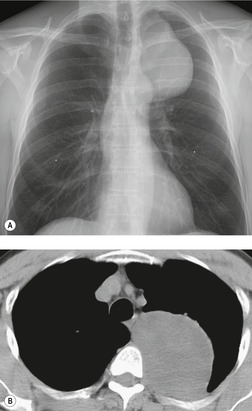
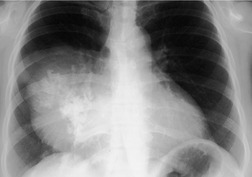
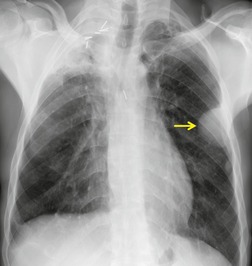
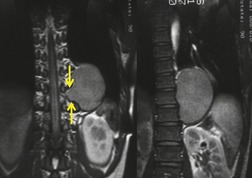
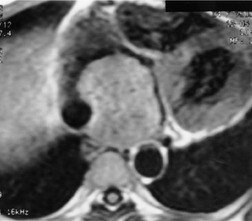
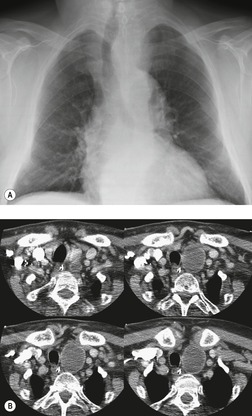
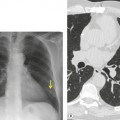
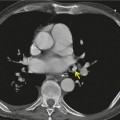
Esophageal duplication cysts are uncommon. They may present in adults or children. 42 The cysts are located in the middle or posterior mediastinum, have muscular coats, and contain mucosa that resembles esophagus, stomach, or small intestine. Esophageal duplication cysts usually occur within the wall, or are adherent to the wall, of the esophagus, are either spherical or tubular in shape, and are usually located along the lateral aspect of the distal esophagus.43. and 44. Many cysts are clinically silent and are first discovered as asymptomatic masses on chest imaging. The remainder manifest with symptoms of dysphagia or chest pain or symptoms due to compression of adjacent structures. 42 Ectopic gastric mucosa in the cyst may cause bleeding into the cyst or perforation of the cyst and the cyst may become infected.
On chest radiographs, esophageal duplication cysts manifest as well-defined round or lobular masses in the middle or posterior mediastinum (Figs 14.1 and 14.2).43. and 44. The masses are usually solid, unless they are infected and contain air. Calcification is rarely detected in the cyst walls. On CT, the cysts manifest as round or tubular water attenuation masses, usually in close proximity to the esophageal wall. These features are similar to those seen in cases of bronchogenic cysts, except that the wall of the esophageal duplication cyst may appear thicker and the mass may have more of a tubular shape than the typical bronchogenic cyst (Fig. 14.2).42.45.46. and 47. On barium swallow examination, the cyst may manifest as either an intramural or extrinsic mass. 42 Although esophageal duplication cysts are usually of water attenuation on CT (Fig. 14.1), some contain proteinaceous fluid or blood and thus appear as soft tissue masses.43. and 44. On MRI, the cysts have similar signal intensity characteristics to bronchogenic cysts, being of variable signal intensity on T1-weighted images, depending on intracystic content, and of markedly increased signal intensity on T2-weighted images (Figs 14.1 and 14.2). 48 Endoscopic ultrasonography can be used to diagnose and treat esophageal duplication cysts. 49
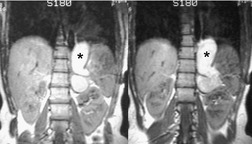 |
| Fig. 14.2 (Courtesy of M Rosado-de-Christenson, MD, Columbus, OH, USA.) |
Pericardial cysts
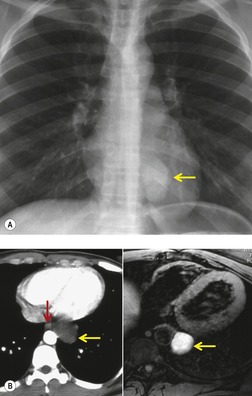
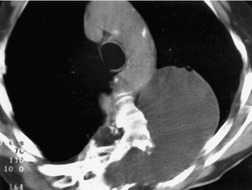
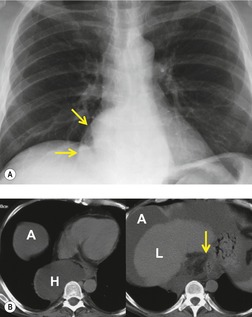
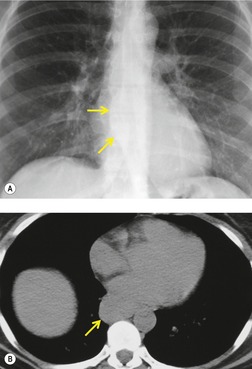
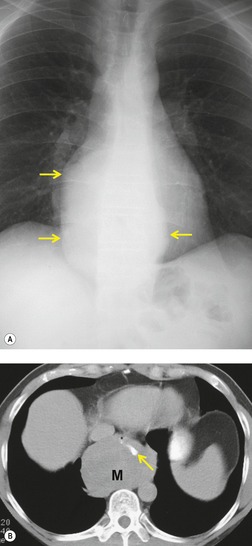
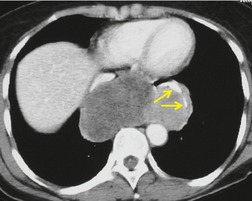
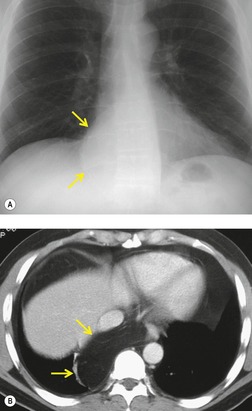
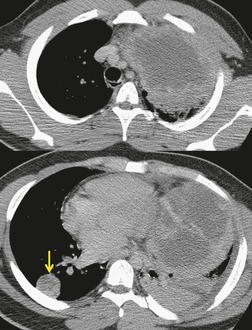
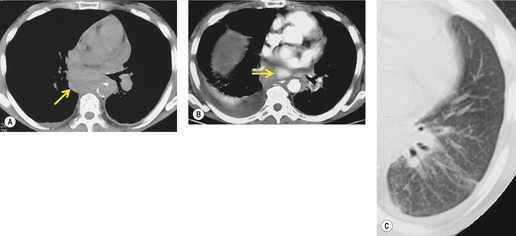
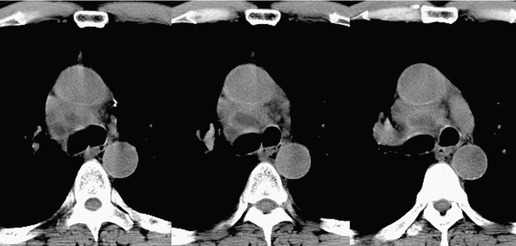
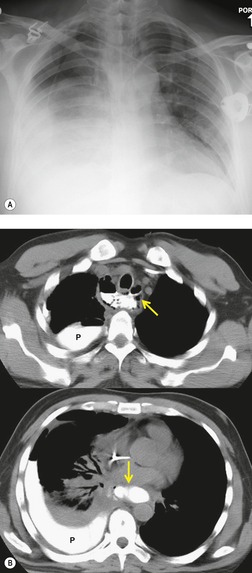
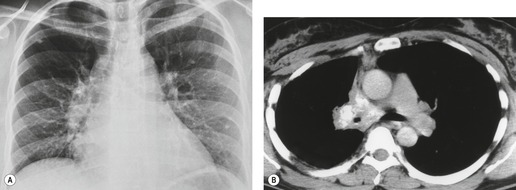
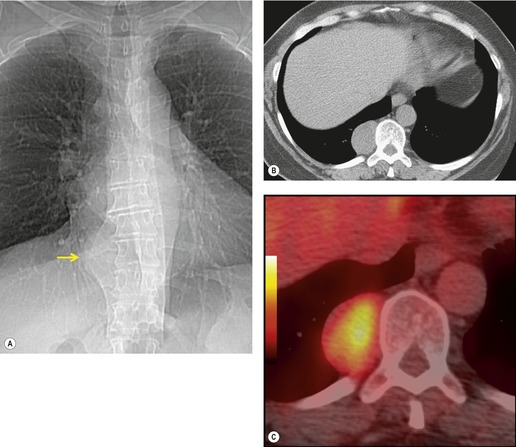
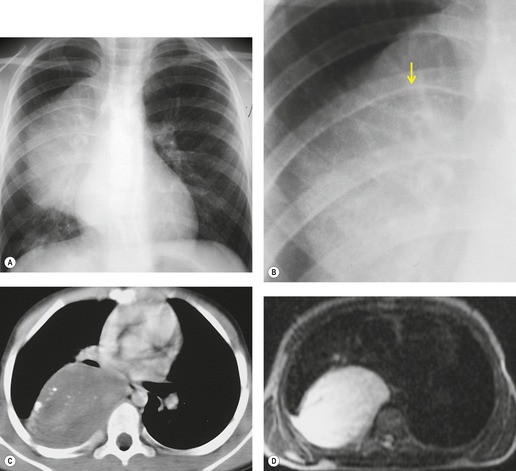
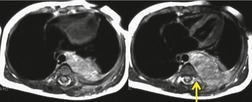
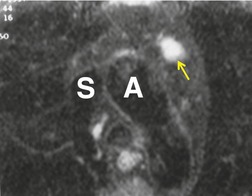
Pericardial cysts are anomalous outpouchings of parietal pericardium, but only rarely have an identifiable communication with the pericardial sac. Pericardial diverticula are related anomalies of the visceral pericardium that communicate with the pericardial space. 50 Rapid change in size, particularly a decrease in size, suggests a pericardial diverticulum rather than a pericardial cyst. 51 The cysts contain clear yellow fluid. The interior is usually unilocular but can be trabeculated. In one series, 20% of cases examined pathologically were multilocular, 52 though in another large series of 72 patients, only one pericardial cyst was truly loculated. 53 The wall of the cyst is composed of collagen and scattered elastic fibers lined by a single layer of mesothelial cells. 52 Most affected patients are asymptomatic at presentation, but in series derived from surgical case material, symptoms such as chest pain, cough, and dyspnea are reported in up to a third of patients.38.52. and 53.
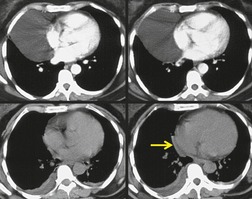
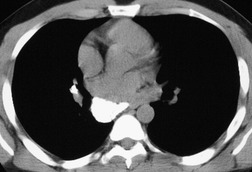
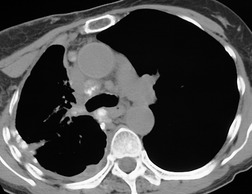
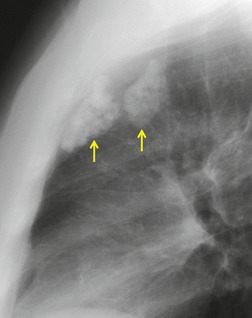
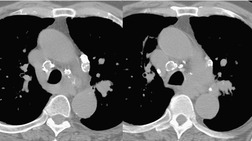
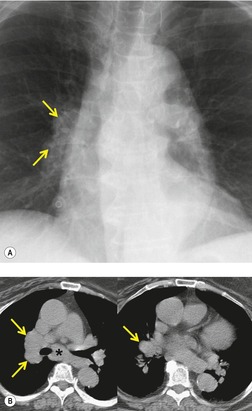
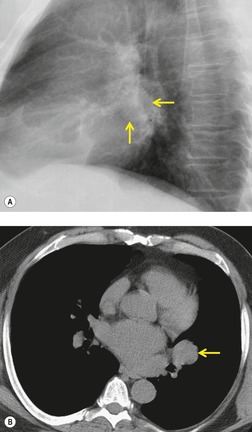
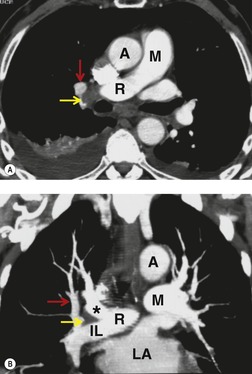
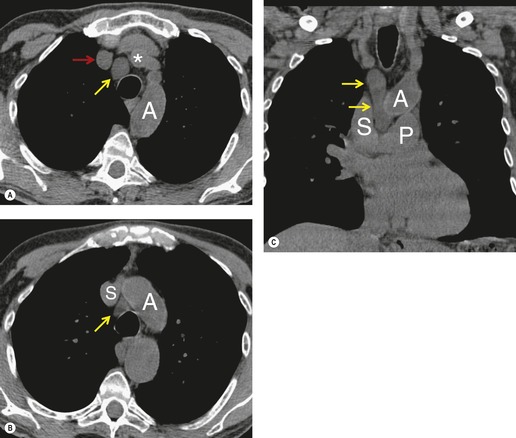
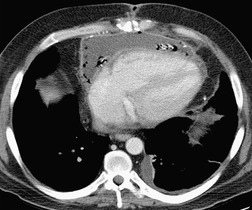
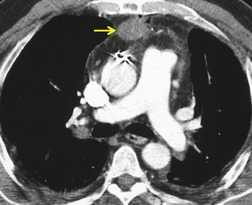
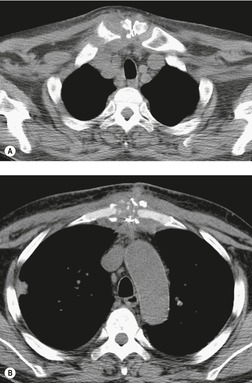
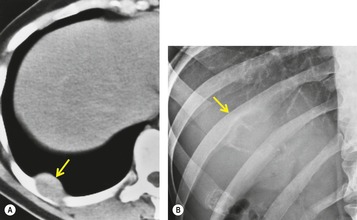
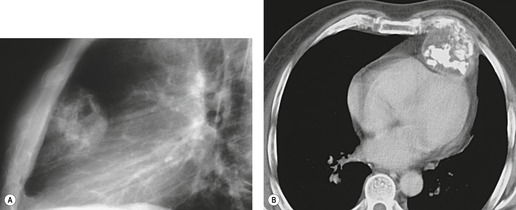
There is a strong predilection for the anterior cardiophrenic angles and the cysts typically contact the heart, the diaphragm, and the anterior chest wall. They are more frequent on the right than on the left. In one large series of 72 patients with pericardial cysts, 37 (51%) were in the right cardiophrenic angle (Figs 14.3 and 14.4) and 17 were in the left. 53 The remaining 18 arose higher in the mediastinum (Fig. 14.5), and 11 extended into the superior mediastinum. In a review of chest radiographs of 41 patients with pericardial cysts, the right-to-left ratio was 4 : 3. 52
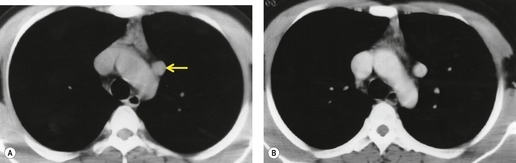
On radiographic studies, the cysts are seen as smooth round or oval well-defined masses in contact with the heart (Fig. 14.3, Fig. 14.4, Fig. 14.5 and Fig. 14.6). Occasionally, the cysts may be large enough to compress adjacent structures such as heart. 54 A pointed oval shape has been observed in some cases.52.55. and 56. Calcification is exceptional on chest radiographs. On CT, the cysts are usually homogeneous water attenuation masses with thin or imperceptible walls (Figs 14.4 and 14.5). The cyst contents should not enhance after administration of intravenous contrast. 57 Soft tissue attenuation pericardial cysts are quite rare. 55 The size of the cysts is quite variable, with very large cysts occasionally reported (Figs 14.3 and 14.4).52. and 53. Ultrasonography can be used to demonstrate the cystic nature of these lesions. MRI shows the mass to be homogeneous and of low signal intensity on T1-weighted images and of high signal intensity on T2-weighted images (Fig. 14.6), similar to water. 32 Cyst puncture and aspiration can be diagnostic in difficult cases or therapeutic in symptomatic patients, although the frequency of recurrence after such intervention is unknown (Fig. 14.4). 58
Neurenteric cysts
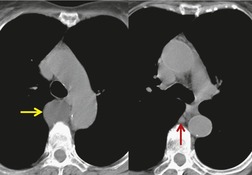
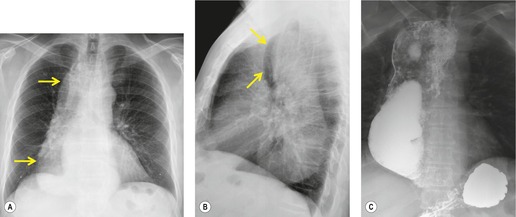
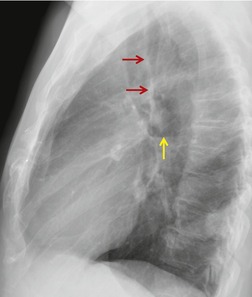
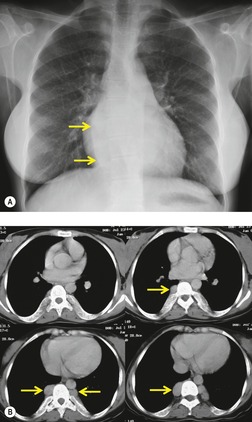
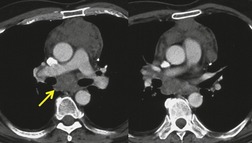
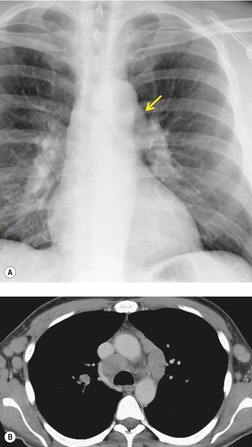
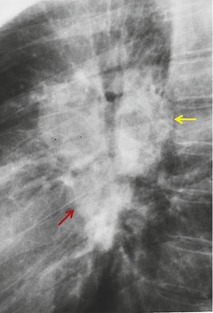
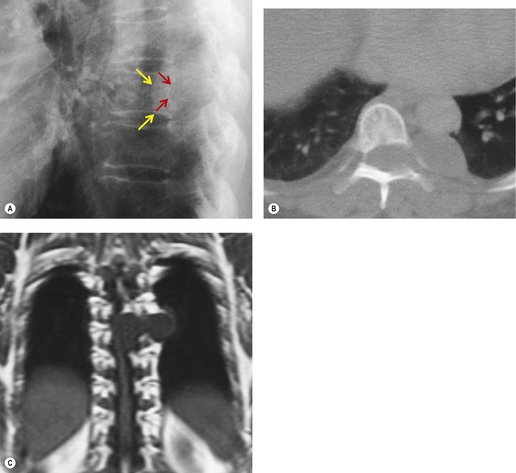
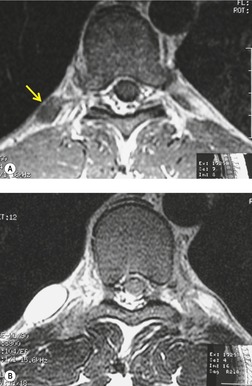
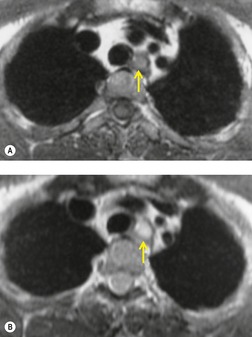
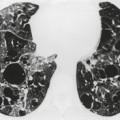
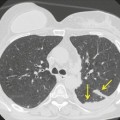
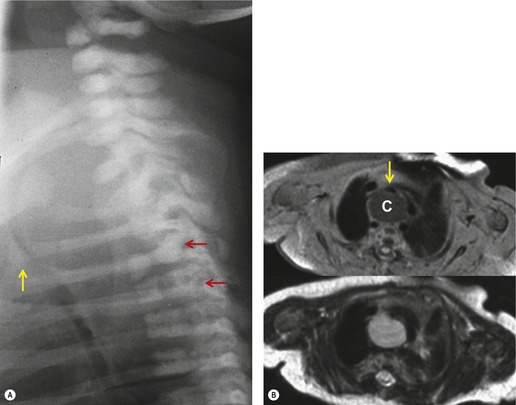
Neurenteric cysts result from incomplete separation of endoderm from notochord, resulting in a diverticulum of endoderm. Neurenteric cysts are pathologically identical to esophageal duplication cysts and usually have either a fibrous connection to the spine or an intraspinal component. 59 These cysts are typically associated with vertebral body anomalies such as hemivertebra, butterfly vertebra or spina bifida that occur at or above the level of the cyst. Most neurenteric cysts occur in the posterior, rather than middle, mediastinum, and usually above the level of the carina. 38 Neurenteric cysts are quite rare.14.38. and 60.
Radiographically (Fig. 14.7),61. and 62. neurenteric cysts are round, oval, or lobulated masses of water density situated in the posterior mediastinum or paravertebral region. Their cystic nature can be demonstrated by ultrasonography. The CT and MRI appearance of these lesions (Fig. 14.7) is similar to that of other foregut cysts. 44 MRI is useful for optimally demonstrating the extent of spinal abnormality and degree of intraspinal involvement. Because the cysts may communicate with the subarachnoid space, CT myelography can also be diagnostic.
 |
| Fig. 14.7 (Courtesy of Lane Donnelly, MD, Cincinnati, OH, USA.) |
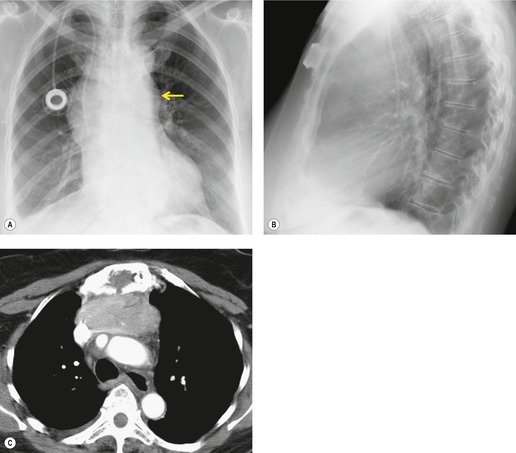
Mediastinal pancreatic pseudocyst
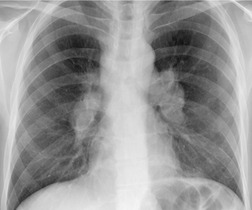
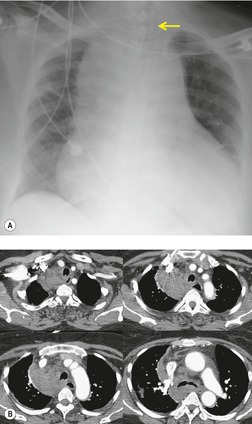
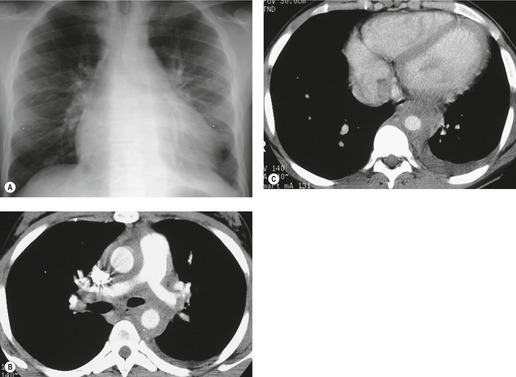
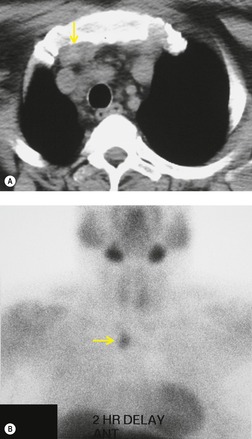
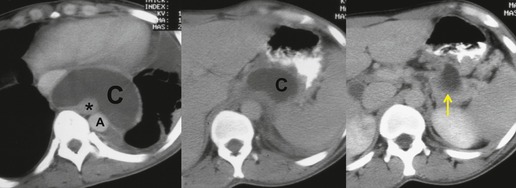
On rare occasions, a pancreatic pseudocyst extends into the mediastinum.63. and 64. Most affected patients are adults and have clinical features of chronic pancreatitis. In children, the usual etiology is trauma. 65 Radiographically, most patients have either bilateral or left-sided pleural effusions. 66 The mediastinal component of the pseudocyst is almost always in the middle and posterior mediastinum, having gained access to the chest via the esophageal or aortic hiatus. The pseudocyst in many instances, therefore, deforms the esophagus. CT is the optimum method of demonstrating the full extent of these pseudocysts. 67 CT shows a thin-walled cyst containing fluid within the mediastinum in continuity with the pancreas (Fig. 14.8), as well as any peripancreatic fluid collections. 68 Magnetic resonance cholangiopancreaticography (MRCP) has been used to successfully diagnose a mediastinal pancreatic pseudocyst. 69 The cyst may, on rare occasion, rupture into the pericardium resulting in tamponade. 70 Hemothorax and esophagobronchial fistula have also been reported as complications of mediastinal pseudocyst. 71 Mediastinal pseudocysts have been successfully treated by endoscopic ultrasonography.72. and 73.
 |
| Fig. 14.8 (Courtesy of May Lesar, MD, Bethesda, MD.) |
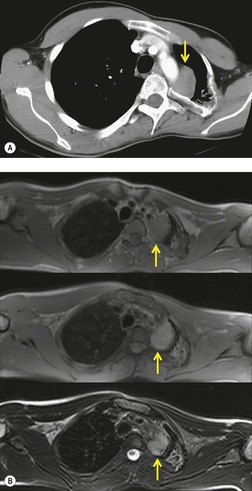
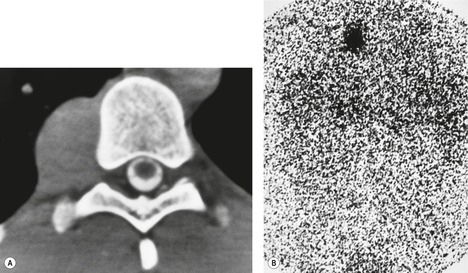
Lateral thoracic meningocele
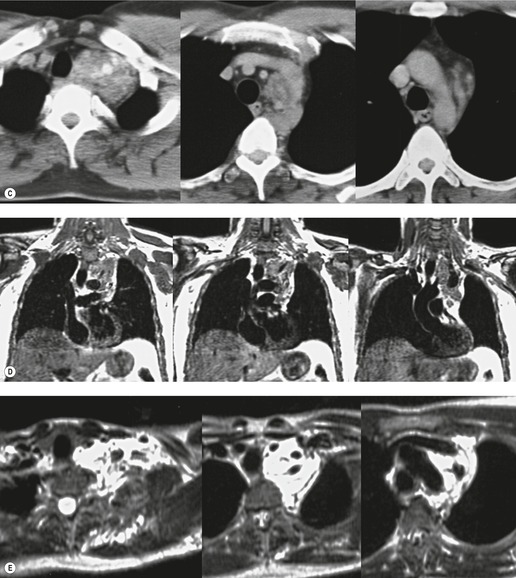
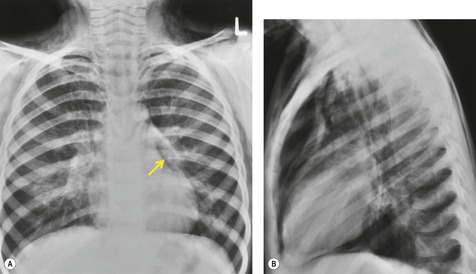
Intrathoracic meningoceles are protrusions of spinal meninges through the intervertebral foramina. 74 They are usually detected in patients between 30 and 60 years of age as asymptomatic masses on chest radiographs. They are rarely associated with pain or neurological abnormalities. 75 Approximately two-thirds of cases occur in association with neurofibromatosis. 75 On rare occasions, multiple or bilateral intrathoracic meningoceles are encountered.76. and 77.
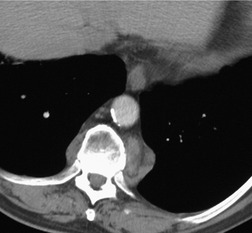
On chest radiographs, 78 these lesions manifest as well-defined paravertebral masses, usually associated with scalloping and deformity of the adjacent ribs, pedicles, or vertebral bodies. Enlargement of the adjacent intervertebral foramen is an important diagnostic feature. Kyphoscoliosis is often present. These findings are identical to those seen in patients with so-called ‘dumbbell’ nerve sheath tumors – a diagnostic problem that is complicated by the fact that both conditions are so frequently associated with neurofibromatosis. CT79. and 80. better demonstrates these features and also shows that the ‘mass’ is of water attenuation, since the bulk of the lesion consists of cerebrospinal fluid (Fig. 14.9). If CT is performed with intrathecal contrast medium, the contrast will enter the meningocele, confirming the diagnosis. Similarly, uniform cerebrospinal fluid (CSF) signal is seen throughout the lesion on MRI. 79
Lymphoceles and thoracic duct cysts
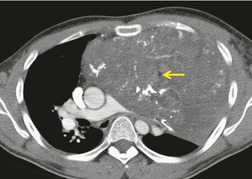
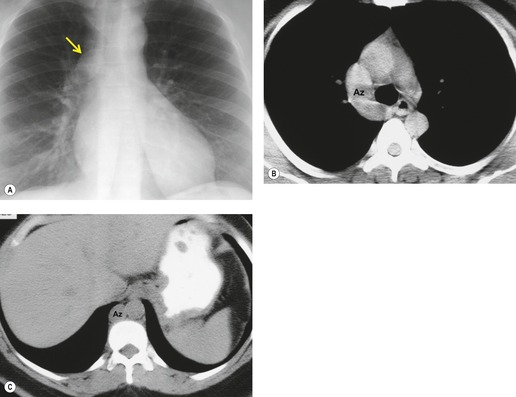
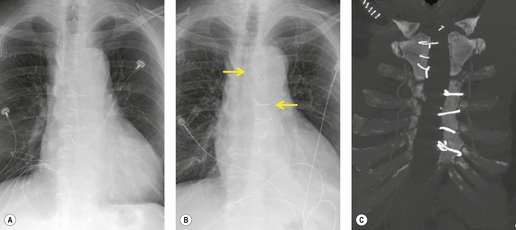
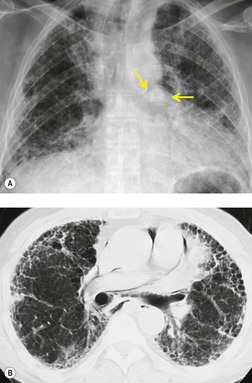
Thoracic lymphoceles are usually due to trauma and are discussed on page 1140. Mediastinal thoracic duct cysts are extremely rare lesions that may be due to either congenital or degenerative weaknesses in the wall of the thoracic duct. 37 The cysts can occur anywhere along the course of the thoracic duct, but have also been reported in the neck. 81 Very large cysts are reported. 82 In a review of 30 reported cases, approximately half of affected patients were asymptomatic. 83 The remainder presented with symptoms such as chest pain, dysphagia, and dyspnea due to compression of adjacent structures.83.84. and 85. CT usually shows a homogeneous mass of water attenuation along the course of the thoracic duct (Fig. 14.10). 83
Desmoid tumor of the mediastinum
Desmoid tumors, also known as aggressive fibromatosis, are locally invasive tumors of fibrous origin that primarily involve the soft tissues of the extremities, neck, and trunk. Histopathologically, they are characterized by a proliferation of fibrous tissue that falls within a spectrum that ranges from benign scar tissue to high-grade fibrosarcoma. 86 The tumors frequently show extensive local invasion, have a high rate of local recurrence after treatment, and can result in significant morbidity. 86 Distant metastases are, however, rare. 87 Desmoid tumors may arise in areas of previous trauma or surgery. 88
Desmoid tumors of the mediastinum and chest wall are rare (Fig. 14.11).88.89.90.91.92.93.94. and 95. On chest radiographs, thoracic desmoid tumors manifest as soft tissue masses that may cause a localized periosteal reaction or cortical erosion of adjacent bone. On noncontrast CT, the mass is usually homogeneous and of the same attenuation as skeletal muscle. 93 On enhanced CT, the lesions are often more heterogeneous and may become hyperattenuating to muscle or show areas of necrosis. 92 Desmoids are usually heterogeneous and of variable signal intensity compared with muscle on both T1- and T2-weighted MR images. 96 As such, there are no particular imaging features to distinguish desmoid tumors from other soft tissue masses in the mediastinum or chest wall.97. and 98. The lesions may be quite invasive, however, infiltrating in and around the great vessels or extending through the diaphragm to involve the abdomen, making complete resection impossible.99. and 100. The lesions are frequently hypervascular at angiography. 101 FDG-PET characteristics of desmoid tumors are not well described. In one small series, the degree of FDG uptake seemed to correlate with aggressiveness of the lesion and propensity for recurrence after resection. 102
Diaphragmatic hernia
Herniation of mesenteric fat or abdominal viscera through congenital or acquired defects in the diaphragm is a common cause of a mediastinal abnormality on chest radiograph or CT. Only hernias through the esophageal hiatus are discussed here. Hernias through the foramina of Bochdalek and Morgagni are discussed on pages 1104–1112.
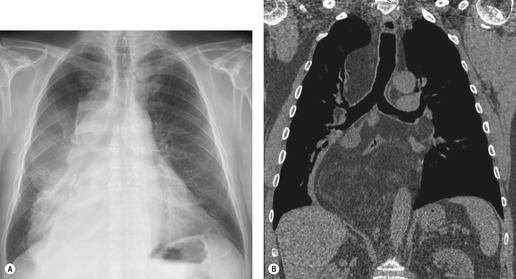
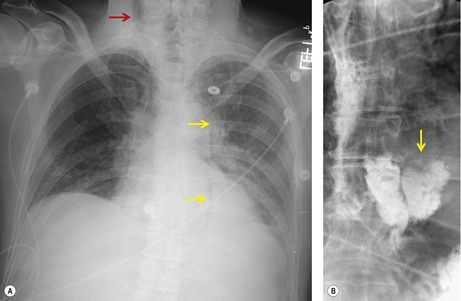
Hiatal hernia
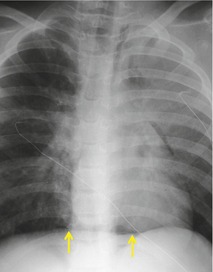
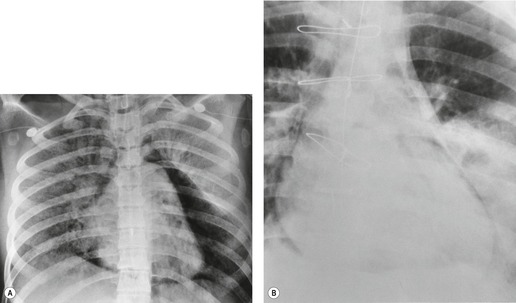
Hiatal hernias are frequent incidental findings on chest radiographs and CT. Pain as a result of gastroesophageal reflux or anemia due to upper gastrointestinal bleeding may be presenting complaints. On chest radiography, they produce a smooth, focal widening of the posterior junction anatomy extending down to the diaphragm. Varying amounts of fat surround the hernia itself, and in most instances some air can be appreciated within the hernia and there may be a visible air–fluid level (Fig. 14.12). When air is present within the hernia, a definitive diagnosis can be made by chest radiography. In the absence of air, however, the differential diagnosis includes other lower paraesophageal masses including lymphadenopathy, and CT or barium swallow examination may be required for confirmation.
At CT, the esophagus can be traced down into the hernia, and air (and contrast material) within the lumen usually enables the diagnosis to be made without difficulty (Fig. 14.12). The fat surrounding the hernia may be a striking feature. Hiatus hernias can be huge and may contain a major portion of the stomach. With large paraesophageal hernias, the stomach not infrequently undergoes organoaxial rotation. 103
On occasion, ascitic fluid under tension may herniate into the mediastinum at the gastroesophageal junction, usually contained by the parietal peritoneum. 104 This so-called ‘communicating thoracic hydrocele’ can occur in the absence of a hiatal hernia and manifests as a mass on chest radiographs. 104 Because the fluid freely communicates with the abdominal cavity, the ‘mass’ may spontaneously disappear. CT shows a water-attenuation middle mediastinal mass closely associated with the esophageal hiatus in a patient with ascites (Fig. 14.13). 105
Esophageal lesions
Various lesions of the esophagus (Box 14.6) can manifest as mediastinal masses, including esophageal dilatation (including achalasia), esophageal duplication cysts (see above), esophageal diverticula, mucoceles, 106 and esophageal neoplasms.107. and 108.
Box 14.6
Diffuse dilatation
• Motility disorder
– Achalasia
– Postvagotomy syndrome
– Chagas disease
– Scleroderma
– Systemic lupus erythematosus
– Presbyesophagus
– Diabetic neuropathy
– Esophagitis
• Distal obstruction
– Carcinoma
– Stricture
– Extrinsic compression
– Mucocele (after distal and proximal exclusion)
• Destruction of the myenteric plexus by tumor (pseudoachalasia)
Esophageal duplication cysts
Esophageal diverticula
Esophageal neoplasms
• Carcinoma
• Stromal tumors
Diffuse dilatation of the esophagus can occur as a result of motility disorders, distal obstruction, or destruction of the myenteric plexus by tumor at the esophagogastric junction. 109 Massive, radiographically evident, esophageal dilatation is most often caused by achalasia or, in some parts of the world, Chagas disease. 110 Achalasia is caused by failure of relaxation of the lower esophageal sphincter. The esophagus can dilate to enormous size in affected patients. Esophageal dilatation is usually best appreciated on the lateral view where the fluid-filled, dilated esophagus displaces the trachea and carina forward (Fig. 14.14). 111 In healthy individuals, the lung usually invaginates posterior to the right half of the trachea, resulting in a thin stripe of soft tissue along the posterior tracheal wall (the posterior tracheal stripe, see Chapter 2). 112 When the esophagus is dilated, the esophagus displaces this lung and the posterior tracheal stripe may appear thickened on the lateral radiograph (Fig. 14.15). This thickened stripe is due to the combined thickness of the trachea and esophageal walls, contained fluid in the dilated esophagus, and, sometimes, periesophageal lymphatic involvement by tumor. 113 The specificity of this finding in isolation is poor, as the stripe can appear thickened in normal patients due to interposition of collapsed normal esophagus between lung and trachea. However, if this sign is seen in association with anterior bowing of the trachea and anterior displacement of the carina, then esophageal dilatation or mass can be confidently diagnosed. The diagnosis of achalasia is further suggested by absence of air in the expected location of the stomach bubble on the frontal radiograph and an air–fluid level within the dilated esophagus.
Double exclusion of the esophagus (distal and proximal) with either colonic or gastric bypass is a surgical procedure occasionally used to treat patients with severe congenital, inflammatory, or neoplastic conditions when esophageal resection is not possible (e.g. lye-induced stricture). In rare cases, the excluded esophageal remnant fills with mucus and dilates, often to large size, resulting in compressive symptoms.106.114. and 115. The diagnosis is made when a tubular mass containing either fluid or proteinaceous material is seen on CT in the characteristic location of the esophagus, in a patient with a history of esophageal exclusion.
Carcinoma of the esophagus, the most common neoplasm of the esophagus, is only occasionally detected as a focal mediastinal mass on chest radiography (Fig. 14.16). Instead, the most frequent finding in affected patients is proximal dilatation of the esophagus, which may be accompanied by recognizable thickening of the esophageal wall. Esophageal dilatation due to an obstructing lesion such as carcinoma is rarely as severe as that seen in patients with achalasia.
Submucosal esophageal neoplasms (gastrointestinal stromal tumors, leiomyomas, leiomyosarcomas) may grow to substantial size without causing dysphagia and may, therefore, present first as an asymptomatic mediastinal mass. 116 Leiomyomas are the most common esophageal stromal tumor (Fig. 14.17). 117 The esophagus is an uncommon location for gastrointestinal stromal tumors (Fig. 14.18). Less than 10% of gastrointestinal stromal tumors are found in the esophagus.117.118.119. and 120. The diagnosis of an esophageal stromal tumor is suggested at barium swallow examination by observing the characteristic signs of an intramural extramucosal mass. On CT, esophageal leiomyomas manifest as smooth, round or ovoid, homogeneous masses (Fig. 14.17) that enhance following administration of intravenous contrast material. The mass is typically inseparable from the esophagus. The esophagus is usually not dilated above the level of the tumor. The absence of proximal esophageal dilatation is an important feature that helps differentiate a stromal tumor such as a leiomyoma from esophageal carcinoma. Leiomyosarcomas and large gastrointestinal stromal tumors (Fig. 14.18) tend to be more heterogeneous on CT. 121 FDG-PET-CT imaging has been primarily used to evaluate known or suspected esophageal mucosal malignancies, 122 but may also be useful for assessment of malignant stromal tumors. 123
Fat-containing lesions of the mediastinum
There are many fat-containing lesions of the mediastinum (Box 14.7), including lipomatosis, mature teratoma (see section on germ cell tumors), thymolipoma (see section on thymic lesions), fatty neoplasms, hernias, and extramedullary hematopoiesis. 124
Box 14.7
• Mediastinal lipomatosis
– Obesity
– Cushing disease
– Corticosteroid therapy
• Neoplasms of fat tissue
– Lipoma
– Lipoblastoma
– Hibernoma
– Liposarcoma
• Fat-containing tumors
– Teratoma
– Thymolipoma
• Herniation of abdominal fat
• Extramedullary hematopoiesis
Mediastinal lipomatosis
Excessive deposition of fat may result in mediastinal widening, a condition known as mediastinal lipomatosis.125. and 126. When associated with generalized obesity, mediastinal lipomatosis does not usually pose a diagnostic problem. However, in patients on steroid therapy or in those with Cushing disease, focal collections of histopathologically normal, but unencapsulated, fat can deposit in many sites, including the mediastinum, and simulate mass lesions on chest radiographs.127.128. and 129. Furthermore, a similar phenomenon is occasionally encountered in patients with normal steroid hormone levels.130. and 131.
On chest radiographs, mediastinal lipomatosis usually manifests as smooth, diffuse widening of the superior mediastinum (Fig. 14.19). There is usually no mass effect upon the trachea or other mediastinal structures. In addition to findings of mediastinal fat deposition, chest radiographs also usually show a symmetric increase in extrapleural fat and the costophrenic angle fat pads may be enlarged as well. When mediastinal fat deposition is symmetric and diffuse, the radiographic appearance is characteristic enough to pose no significant diagnostic difficulty (Fig. 14.19). On the other hand, when deposition is asymmetric or more focal, CT may be required to exclude a soft tissue mass (Fig. 14.20). This may be the case in patients with lymphoma who are being treated with corticosteroids. At CT, the uniform low attenuation of fat is diagnostic (Figs 14.19 and 14.20).130.132. and 133.
A rare inherited condition termed multiple symmetric lipomatosis, also known as Madelung disease or Lanois–Bensaude syndrome, radiologically resembles mediastinal lipomatosis. In this condition, multiple masses of benign fat tissue proliferate at various sites including the mediastinum. 134 Unlike mediastinal lipomatosis, however, these masses occasionally compress mediastinal structures such as the trachea135 or larynx. 136 Although the pathogenesis is unclear, the disease may be due to defective regulation of brown fat.137.138.139. and 140.
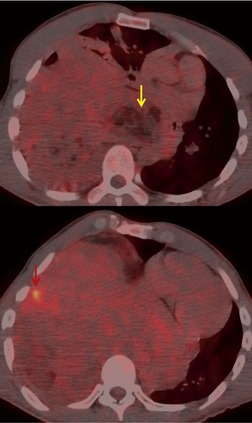
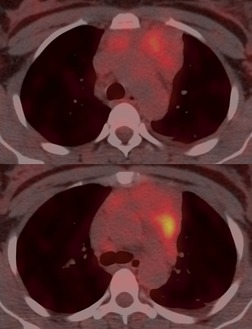
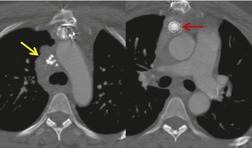
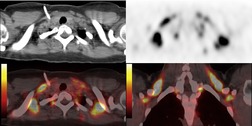
Increased use of combined FDG-PET-CT imaging in oncology patients has led to the recognition that metabolically active fat, known as brown fat, can accumulate FDG and lead to false-positive interpretations. 141 Brown fat is more commonly found in young patients and in women. It is most commonly seen in the neck and paravertebral regions, but can also be found in the mediastinum. 142 Careful correlation between the CT image and foci of FDG uptake should prevent misinterpretation (Fig. 14.21).
 |
| Fig. 14.21 (Courtesy of Terry Wong, MD, Durham, NC, USA.) |
Fatty tumors of the mediastinum
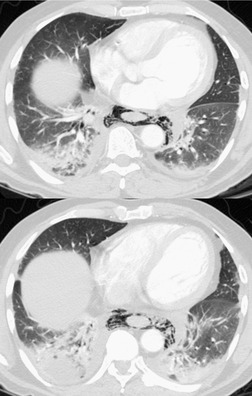
Mediastinal tumors of fatty origin are uncommon, accounting for less than 1% of 1064 surgically proved mediastinal masses. 14 On chest radiographs, both benign and malignant fat-containing tumors manifest as well-defined, round or oval, mediastinal masses. 143 Benign lipomas usually do not compress surrounding structures unless they are very large. Quinn et al. 144 reported one unusual case where a mediastinal lipoma extended into the spinal canal and caused pressure deformity of the adjacent bones. Large mediastinal lipomas may mold so completely to mediastinal contours as to simulate the appearance of an enlarged heart. 145 CT of mediastinal lipomas usually shows a homogeneous mass of fat attenuation. The lesion may contain a very few strands of soft tissue or septa, but these should be quite thin (<1.0 mm).144. and 146. Lipoblastomas are benign fat-containing tumors that usually occur in childhood.147. and 148. On CT, both fat and soft tissue components are seen.29.149. and 150. Occasionally, the amount of fat seen on CT is relatively small and the mass is primarily of soft tissue attenuation. 151 Hibernomas are very rare benign tumors that contain brown fat. 152 The CT features of mediastinal hibernomas are the subject of case reports only.152. and 153. Based upon these reports, the masses appear as mixed fat, water, and soft tissue attenuation. The amount of fat that is identifiable seems to be variable. 154 As hibernomas contain metabolically active brown fat, they can show considerable FDG uptake on FDG-PET imaging.152. and 155. Angiolipoma and myelolipomas are also very rare benign tumors that can occur in the mediastinum. They also manifest as masses of mixed soft tissue and fat attenuation on CT, and are thus indistinguishable from liposarcoma.156. and 157.
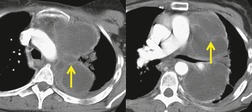
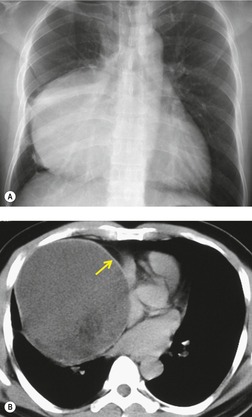
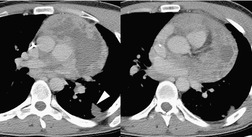
Liposarcomas are malignant fat-containing tumors that occur more often in the anterior than the posterior mediastinum (when they occur in the mediastinum).158. and 159. Most affected patients are middle-aged adults who present with symptoms of chest pain and dyspnea. The masses are typically large and may diffusely infiltrate the mediastinum. Well-differentiated liposarcomas may have large amounts of fat admixed with soft tissue components on CT or MR images (Fig. 14.22). 124 On occasion, the lesions may be almost completely fatty with only a minimal soft tissue component. 160 High-grade tumors usually do not have significant amounts of fat demonstrable on CT or MRI; instead, these lesions manifest as infiltrative masses of heterogeneous soft tissue attenuation (Fig. 14.22) or signal intensity. 158 Myxoid liposarcomas of the mediastinum may contain regions of near water attenuation on CT (Fig. 14.23) and may have calcified stroma. 161
It can be difficult to confidently differentiate benign lipomas from well-differentiated liposarcomas of the mediastinum. This question has not specifically been addressed in regard to mediastinal lesions. However, numerous studies of peripheral fatty tumors suggest that large size (>10.0 cm), male sex, fat content less than 75% of the lesion, thick internal septa (>1.0 mm), and nodular or globular soft tissue components favor liposarcoma.162.163.164. and 165. Areas of high signal intensity on fluid-sensitive MRI sequences are also a suggestive feature of liposarcoma. 162 FDG-PET-CT imaging features of mediastinal liposarcomas have not been reported. Based on experience with extremity tumors, however, the degree of FDG uptake likely correlates with likelihood of malignancy, histopathologic grade, and aggressiveness of the lesion (Fig. 14.24).166. and 167. When in doubt, histopathologic sampling is required.
Herniation of abdominal fat
Herniation of omental or perigastric fat is a common cause of a localized fatty mass in the mediastinum. The fat may herniate through the esophageal hiatus, the foramen of Morgagni, or the foramen of Bochdalek. Such herniations are usually readily diagnosed on chest radiographs because of their characteristic locations. The masses are of fat attenuation or signal intensity on CT29 or MRI168 and may contain linear or nodular foci due to contained omental vessels (Fig. 14.25).
Extramedullary hematopoiesis
Extramedullary hematopoiesis in potential blood-forming organs such as the liver, spleen, and lymph nodes is common in patients with severe anemia. Thoracic manifestations are rare and usually consist of paravertebral soft tissue masses, although pulmonary parenchymal involvement has been described. 169 The masses are caused by extrusion of the marrow through the thinned cortex of the posterior ribs. Histopathologically the masses resemble splenic tissue with hematopoietic elements mixed with fat. The masses themselves are usually asymptomatic, though paraplegia from cord compression may occur.170. and 171. Massive hemothorax secondary to rupture of the hematopoietic masses has been described. 172 The most common conditions that result in extramedullary hematopoiesis are the congenital hemolytic anemias, notably thalassemia, hereditary spherocytosis, and sickle cell disease. 173 However, it may rarely occur in other anemias and may even occur in patients without anemia. 174
Thoracic extramedullary hematopoiesis manifests on chest radiographs,171.174. and 175. CT,175. and 176. and MRI177 as focal paravertebral masses, usually in the lower half of the thorax (Fig. 14.26). The masses are usually well marginated because they are covered by pleura, are bilateral in distribution, contain no calcification, and show no rib destruction. Further foci of extramedullary hematopoiesis can also be seen as subpleural masses adjacent to ribs. These subpleural masses may be continuous or discontinuous with the paravertebral masses. The adjacent bone is usually normal or shows findings of marrow expansion; pressure erosions or bone destruction do not occur. 174 CT is particularly useful for demonstrating the lacelike marrow expansion in the adjacent bones (Fig. 14.26). 175 On CT, the lesions manifest as heterogeneous or homogeneous soft tissue attenuation masses (Figs 14.26 and 14.27). There may be some fat within the mass (Fig. 14.27), 178 but calcification is uncommon. 179 On MRI, the masses are usually heterogeneous with increased signal intensity on T1-weighted images because of contained fat. 177 Radionuclide studies using agents that show erythropoiesis or the reticuloendothelial system may demonstrate activity in the mass,175.180.181. and 182. but can be negative.175. and 183. Diagnosis can also be established by fine needle aspiration biopsy. 184
Germ cell tumors of the mediastinum
Germ cell tumors (Boxes 14.8 and 14.9) account for 10–15% of anterior mediastinal masses and are thought to arise from mediastinal remnants left behind after embryonal cell migration.185.186.187. and 188. The mediastinum is the most common primary site for extragonadal germ cell tumors and mediastinal lesions account for about 60% of all germ cell tumors in adults. Germ cell tumors usually occur in young adults; the mean age at presentation is 27 years.185.186. and 187. Most malignant germ cell tumors (>90%) occur in men. Benign lesions (mature teratoma) occur with equal frequency in men and women. Histopathologic types of germ cell tumors that occur in the mediastinum include teratoma, seminoma, embryonal carcinoma, endodermal sinus tumor, choriocarcinoma, and mixed tumors. 189 Malignant germ cell tumors frequently secrete tumor markers such as hCG, AFP, or lactate dehydrogenase (LDH). These serum markers can be used to diagnose and monitor the progress of the disease. 190 Poor prognostic factors include mediastinal location, metastases at presentation, and degree of elevation of serum tumor markers. 191
Box 14.8
Location
• Usually anterior
• Rarely middle or posterior
Demographics
• Young adults
• 15% of anterior mediastinal masses
• 90% occur in men (malignant form)
• Mature teratomas occur with equal frequency in men and women
Histopathology
• Teratoma
– Mature teratoma
– Immature teratoma
– Immature teratoma – malignant
– Teratoma with additional malignant components
• Seminoma
– Most common pure histology
• Nonseminomatous germ cell malignancy
– Yolk-sac tumor
– Embryonal cell carcinoma
– Choriocarcinoma
• Mixed tumors
Clinical
• May be asymptomatic
• Malignant more likely to present with symptoms
• Malignant associated with serologic markers
– AFP
– hCG
– Lactate dehydrogenase (LDH)
Poor prognostic factors
• Nonseminomatous histology
• Mediastinal location
• Nonpulmonary metastases at presentation
• AFP >10 000 ng/mL
• hCG >50 000 IU/L
Box 14.9
Mature teratoma
• Chest radiography
– Well-circumscribed mediastinal mass
– Typically unilateral
– May contain calcification
• CT
– Appear as uni- or multilocular cystic mass
– Fat
• Seen in 75%
• Predominant feature in 15%
• Fat–fluid level rare, but diagnostic
– Calcification common
• Rimlike
• Internal coarse
– Rupture
– Lesions are more heterogeneous
– Fat in pleural space, pericardium, lung
• MRI
– Complex signal patterns depending on proportion of water, soft tissue, fat, and calcification
Immature teratoma/malignant elements
• Large bulky tumors
• Heterogeneous on CT/MRI
• Invasive
• May or may not contain fat/calcification
Malignant nonteratomatous germ cell tumors
• Seminoma
– Large, lobulated, homogeneous
– May have prominent cystic component
– Fat or calcification rare
– Indistinguishable from lymphoma
– Nodal metastases
• Nonseminomatous
– Larger and more ill-defined borders
– May be bilateral
– Frequently invasive
– Very heterogeneous with areas of necrosis or cysts
– Fat or calcification rare
Teratoma
Teratomas are the most common mediastinal germ cell tumors and are derived from more than one embryonic germ layer. Most mediastinal teratomas arise in cell rests within, or in intimate contact with, the thymus.192. and 193. Teratomas are classified histopathologically as mature or immature. Immature teratomas are further subclassified as immature teratoma, immature teratoma – malignant, or immature teratoma with additional malignant components.194 The term teratocarcinoma is now discouraged. 190Mature teratomas are composed of different tissue types (ectoderm, endoderm, mesoderm), with ectodermal derivatives predominating. 195 The term dermoid cyst is commonly used when the tumor contains primarily ectodermal components such as skin, sebaceous material, hair and calcification.189. and 196. Such lesions are typically unilocular; multilocular lesions with intervening solid portions are less common. 14 Tumors with more than 10% immature elements are classified as immature teratoma and are considered potentially malignant. 190Immature teratoma – malignant is such a lesion that develops metastases after diagnosis. Teratomas that contain malignant components such as other malignant germ cell neoplasia, sarcoma, or adenocarcinoma, are termed immature teratoma with additional malignant components and are frequently metastatic at presentation.190.194. and 197. Such tumors have a very poor prognosis.
Mature teratomas account for 70% of germ cell tumors in childhood and 60% of mediastinal germ cell tumors in adults. Mature teratomas occur most frequently in children and young adults.14.185. and 198. About half of affected patients are asymptomatic at presentation, with the lesion being detected on chest radiographs obtained for other purposes. In the remainder, symptoms due to local compression, rupture, or infection occur. The most common presenting complaints are chest pain, productive cough, dyspnea, or fever. 199 Rarely, affected patients present with pneumonia, hemoptysis, or the superior vena cava syndrome. Trichoptysis (the expectoration of hair) is a dramatic, but extremely rare, symptom that occurs when the lesion ruptures into the airway.14. and 189. Patients with mature teratoma usually have normal serum levels of β-hCG and AFP. Complete resection is the treatment for mature teratomas and usually results in a complete cure. Despite a benign histology, these tumors may be difficult to remove when they are adherent to local structures.
On chest radiographs, mature teratomas manifest as well-defined, rounded, or lobulated masses that usually project to one side of the midline (Fig. 14.28). 14 They most commonly occur in the anterior mediastinum, typically in the prevascular space. On occasion, they occur in the posterior mediastinum or the lung parenchyma itself.14.186.199.200.201. and 202. The lesions tend to grow very slowly, but may increase in size rapidly if intratumoral hemorrhage occurs. Calcification, ossification, or even teeth199. and 200. may be visible on chest radiographs (Fig. 14.29) and, occasionally, sufficient fat is present within the lesion to be detectable radiographically.
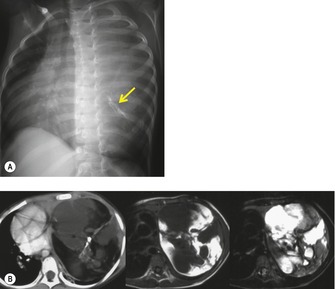 |
| Fig. 14.29 (Courtesy of Lane Donnelly, MD, Cincinnati, OH, USA.) |
The CT appearance of mature teratoma is quite variable because it depends upon the content of the lesion (Figs 14.28 and 14.29).203. and 204. Almost all lesions have some areas of water attenuation on CT. Regions of fat attenuation are seen on CT in up to three-quarters of lesions and are the predominant tissue type in 15%. 199 Fat–fluid levels within the lesion, while uncommon, are virtually diagnostic of teratoma (Fig. 14.30).205. and 206. More often, however, the fat is interspersed with regions of water and soft tissue attenuation. A definite cyst wall, which may have curvilinear calcification, may be visible on CT (Fig. 14.28), as is characteristic intralesional calcification (Fig. 14.29). 199
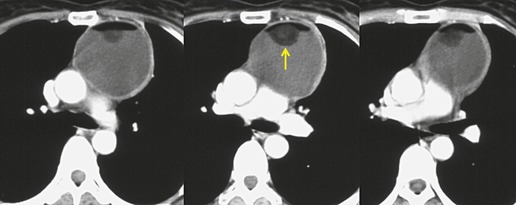 |
| Fig. 14.30 (Courtesy of M. Rosado-de-Christenson, MD, Columbus, OH, USA.) |
On rare occasions, the lesions rupture into the airway, pleural space, or pericardium. When the tumor ruptures into the airway, air may enter the cyst and become visible on imaging examination; severe chemical pneumonitis or lung abscess can also result. 38 In a review of 17 patients with seven ruptured and 10 unruptured teratomas, those lesions that ruptured were noted to be more internally heterogeneous on CT than were those that were not ruptured. 207 Additional findings suggestive of rupture were adjacent consolidation, atelectasis, pleural, or pericardial effusion. 207 In two cases, fat was seen in the lung parenchyma. Rupture into the pericardium208 or pleura can result in the appearance of a fat–fluid level within these spaces on imaging examinations. 209
As is the case with CT, the MRI appearance of mature mediastinal teratomas is quite variable (Fig. 14.29). Because the contents of the cyst are typically rich in proteinaceous fluid, the cystic component of the lesion may be of high signal intensity on T1-weighted images.32.199. and 210. Furthermore, the lesion may be of high signal on T1-weighted images because of fat or hemorrhage. On ultrasonography, the lesions may appear as completely cystic or solid masses or as mixed cystic and solid masses. 211 The pattern on ultrasonography is often quite complex because of intralesional calcification (densely echogenic), hair (hyperechoic dots), and fat (dense echo pattern). 212 One case that showed echogenic floating spherules in the mass has been described. 213
Information regarding FDG-PET imaging features of mediastinal mature teratoma is scant. Based upon limited experience with germ cell tumors in other sites, it is likely that these lesions, unless complicated, show little or no FDG accumulation. 214 Any foci of increased FDG accumulation should suggest a more aggressive lesion, such as immature teratoma, until proven otherwise.
Imaging features of immature teratoma or teratoma with malignant components are less well-described. 190 Masses are typically described as large, invasive, and quite heterogeneous on CT or MRI, and may or may not contain areas of fat or calcification (Fig. 14.31). 190
Malignant nonteratomatous germ cell tumors
In addition to teratomas with malignant features, other types of malignant germ cell tumors that occur in the mediastinum include seminoma, embryonal cell carcinoma, endodermal sinus tumor, choriocarcinoma, and mixed germ cell tumors.215. and 216. Seminoma is the most common pure histopathologic type in men and accounts for 40% of such tumors. 216 However, tumors of mixed histology are more common than pure seminoma. 215 Malignant mediastinal germ cell tumors are usually encountered in young adults and are much more common in men than women. 192 In a review of 103 cases of primary mediastinal seminoma, only five occurred in women. 217 Even mediastinal choriocarcinoma in adults is more common in men than women; in children, the male–female ratio is more evenly distributed. 192 Because of marked differences in prognosis and treatment, nonteratomatous malignant germ cell tumors are frequently grouped as seminoma and nonseminomatous germ cell malignancies.191
Malignant mediastinal germ cell tumors are more frequently symptomatic than mature teratomas.187.190. and 192. Common presenting complaints include cough, dyspnea, and chest pain. 217 Superior venal cava obstruction is reported in up to 10% of affected patients.217. and 218. Weight loss may also be a notable feature. However, between 10% and 30% of affected patients are asymptomatic at presentation, with the mass discovered on routine chest radiographs. 191
Serum levels of hCG and AFP are useful for diagnosis and monitoring of some mediastinal germ cell malignancies.187. and 190. Both hCG and AFP levels are typically normal in cases of pure seminoma; slight elevation in hCG levels are occasionally encountered; elevation of AFP indicates a nonseminomatous component of the tumor. Up to 80% of patients with nonseminomatous germ cell malignancies have elevated levels of AFP and 54% have elevated levels of hCG.187. and 190. There is an association between malignant nonseminomatous germ cell tumors of the mediastinum and hematologic malignancies219. and 220. and up to 20% of affected patients may have Klinefelter syndrome.221. and 222.
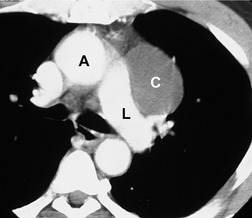
Seminomas typically manifest on chest radiographs as focal, unilateral or bilateral, mediastinal masses (Fig. 14.32). On CT or MRI, they are usually large lobulated masses of homogeneous attenuation or signal intensity, often indistinguishable from lymphoma (Fig. 14.32).187. and 190. Cysts or areas of necrosis may also be seen in association with mediastinal seminoma.187. and 190. Invasion of adjacent structures may occur but calcification is rare. Metastases to regional nodes or lung can occur (Fig. 14.32).
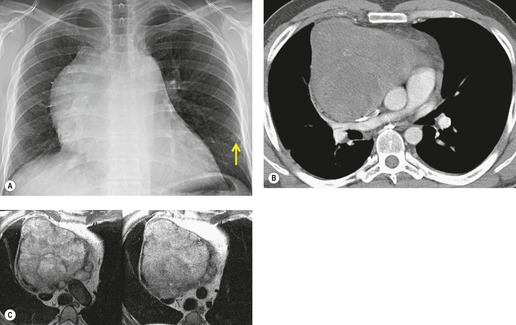 |
| Fig. 14.32 (Courtesy of Jeremy Erasmus, MD, Houston, TX, USA.) |
Nonseminomatous germ cell malignancies usually manifest as large lobular or ill-defined anterior mediastinal masses (Figs 14.33 and 14.34). 190 The mass is typically asymmetric and projects to one side of the thorax.185.223. and 224. Calcification is an exceptional finding on chest radiographs. On CT or MRI, the mass may be quite heterogeneous and may contain cysts or areas of necrosis and hemorrhage (Figs 14.33 and 14.34).225. and 226. These features may be accentuated following administration of intravenous contrast. Coarse tumor calcification is rarely seen on CT.225. and 227. Adjacent mediastinal fat planes are often obliterated and extensive local invasion may be identified. 228 Invasion of the adjacent mediastinal structures, chest wall, and lung, as well as metastases to the regional lymph nodes and distant sites, is common (Figs 14.33 and 14.34). 190
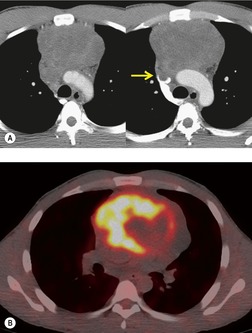
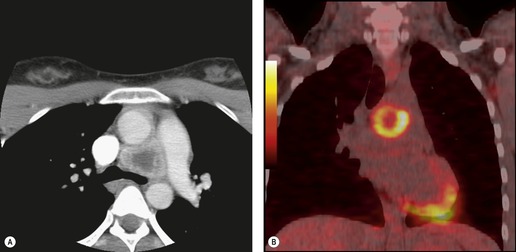
The FDG-PET imaging features of mediastinal germ cell malignancies are not well described. Most of the literature on the subject describes findings in patients with testicular or ovarian primary tumors.11.229.230.231. and 232. Extrapolation of such results to patients with mediastinal primary tumors is difficult, as the nonseminomatous mediastinal tumors tend to behave quite differently from their testicular or ovarian counterparts. Nevertheless, it is likely, based upon these results, that pure teratoma will show little if any increased metabolic activity. 230 Malignant tumors (seminoma or nonseminomatous), on the other hand, should show metabolic activity (Figs 14.33 and 14.35). Results of staging studies with testicular primary tumors are mixed, with one multicenter randomized trial showing only a slight positive benefit for FDG-PET over CT. 229 Marked FDG uptake in a residual mass after treatment, however, does seem to predict viable tumor. 232
A residual mediastinal mass is sometimes seen on CT after successful treatment of a primary mediastinal nonseminomatous germ cell malignancy or after treatment of metastatic disease from a gonadal primary (Fig. 14.36). This mass may be cystic in nature, contain residual mature teratoma, and enlarge with time.233. and 234. This phenomenon is known as the ‘growing teratoma’ syndrome.235.236.237. and 238. The benign or malignant nature of the residual mass cannot be confidently determined based upon the CT appearance alone. However, elevation of serum tumor markers suggests a malignant component. Andre et al. 236 followed 30 patients with growing teratoma syndrome. All 30 lesions were biopsied or resected and 86% of lesions were found to have a mature teratoma component. All but one patient who underwent curative resection survived disease-free. Five of the six patients who had only partial resection developed recurrent disease and one died of progressive tumor. These authors and others233 concluded that complete surgical resection was the treatment of choice.
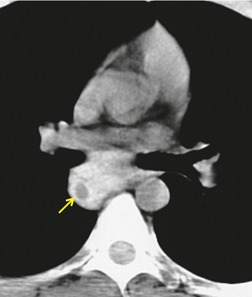 |
| Fig. 14.36 (Courtesy of M Rosado-de Christenson, MD, Columbus, OH.) |
Lymphadenopathy
Causes of lymphadenopathy
Lymphadenopathy (Box 14.10) can be caused by a variety of infectious, inflammatory, and neoplastic conditions. Neoplastic causes include lymphoma, leukemia, and metastatic carcinoma. Lymph node metastases frequently occur in the setting of thoracic malignancies such as lung (Fig. 14.37), esophagus, and breast cancer. Extrathoracic tumors that frequently spread to intrathoracic lymph nodes include renal, testicular, and head and neck malignancies.239. and 240. The most common infections that result in intrathoracic lymphadenopathy are tuberculosis (Fig. 14.38) and fungal disease (particularly histoplasmosis and coccidioidomycosis). Lymph node enlargement is frequent in acquired immune deficiency syndrome (AIDS) patients (see Chapter 6) and can be caused by lymphoma or granulomatous infection. Rare infections such as tularemia, anthrax, and plague can also cause lymphadenopathy. Enlarged nodes in patients with anthrax are characteristically of high attenuation on CT due to extensive hemorrhage. 241 Significant lymphadenopathy is quite uncommon in other infections, particularly bacterial pneumonia, and, when present, suggests an unusual organism or alternative diagnosis.
Box 14.10
Infection
Inflammatory
Neoplasm
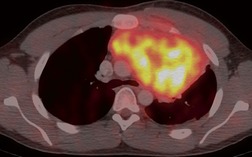
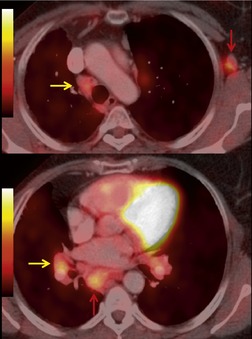
Sarcoidosis is a particularly frequent cause of intrathoracic lymph node enlargement in young adults (Fig. 14.39). When multiple lymph node groups in the hila and mediastinum are symmetrically enlarged in a young patient, sarcoid is the most likely diagnosis. Lymph nodes in patients with sarcoidosis are frequently positive on FDG-PET scans (Fig. 14.40). 242 Lymphoma is the most important differential diagnosis in such patients, but lymphoma is rarely so symmetrically distributed with equal involvement of the hilar and mediastinal lymph node groups (Fig. 14.41). Isolated paracardiac node enlargement is unusual in cases of sarcoid or infection, and are often due to lymphoma or metastatic carcinoma.243. and 244. Lymphoma and sarcoidosis are discussed further in Chapters 13 and 11, respectively.
Reactive hyperplasia is a term used to describe an acute or chronic nonspecific inflammatory response in which both inflammation and hyperplasia are present. Lymph nodes undergo reactive changes whenever challenged by infection, cell debris, or foreign substances. Thus, nodal enlargement due to reactive hyperplasia is seen in nodes draining areas of pulmonary infection, bronchiectasis, and a variety of inflammatory and chronic interstitial lung diseases,245. and 246. and also in nodes draining neoplasms. Reactive hyperplasia is a common cause of false-positive lymph nodes on FDG-PET scans in patients with nonsmall cell carcinoma. 247 In such cases, high FDG uptake has been attributed to overexpression of the glucose transporter-1 enzyme in regions of follicular lymphoid hyperplasia.248. and 249.
Chronic left heart failure is an important and perhaps underrecognized cause of mediastinal lymphadenopathy.250.251.252. and 253. Between 42% and 66% of patients with left heart failure will have enlarged lymph nodes (>1 cm short axis, see below) on CT.250.252. and 253. Most (63%) are in the pretracheal regions and the mean short axis diameter in one series was 1.3 cm. 252 Node margins may be ill-defined and mediastinal fat may be of increased attenuation (‘hazy’) in up to 33%. 250 Presence of enlarged nodes has been shown to correlate with decreased left ventricular ejection fraction.252. and 253. Node enlargement may resolve after treatment for heart failure. 253 The etiology for lymph node enlargement in this setting is unknown. Histopathologic sampling in three patients with congestive heart failure and mediastinal lymphadenopathy showed only sinus histiocytosis. 251 It has been speculated, however, that mediastinal lymph nodes hypertrophy in response to chronic mediastinal edema and lymphatic congestion. 251
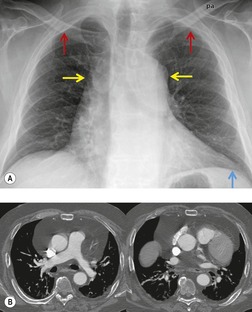
Mediastinal lymph node enlargement is also common in patients with pulmonary fibrosis that occurs either idiopathically254. and 255. or in the setting of collagen vascular disease (Fig. 14.42)245. and 256. or asbestos exposure. 257 In one series, 13 (93%) of 14 patients with usual interstitial pneumonia had enlarged mediastinal lymph nodes on CT. 255 Nodes larger than 2 cm in short axis diameter were seen in three (21%). 255 In another series, enlarged nodes were found on CT in 67% of 175 patients with diffuse infiltrative lung disease. 245 These authors noted that the vast majority of patients had only a few enlarged nodes and that they rarely exceeded 15 mm in short axis diameter. 245 Jung et al. 254 reported enlarged nodes on CT in 86% of 30 patients with pulmonary fibrosis and also found that the number of enlarged nodes correlated with disease severity. Histopathologic analysis of enlarged lymph nodes in patients with fibrosis usually shows reactive change or sinus histiocytosis. 245
Castleman disease
Castleman disease (Box 14.11), also known as giant lymph node hyperplasia or angiofollicular lymph node hyperplasia, is a rare cause of often massive lymph node enlargement in the chest. 285 Although intrathoracic lymph nodes are most commonly affected, nodes at any location can be involved.276. and 286. Extranodal involvement, including spleen and lung parenchyma, can also occur.287.288. and 289.
Box 14.11
Etiology
• Unknown
• Abnormal production of B lymphocyte growth factor (interleukin [IL]-6)
• Associated with human herpes-8 virus (HHV-8) in human immunodeficiency virus (HIV+) individuals
Histopathology
• Hyaline-vascular – 80%
• Plasma cell – 10%
• Mixed lesions
Location
• Middle mediastinum (subcarinal or paratracheal)
• Hila
• Other mediastinal, chest wall, lung parenchyma
Current clinical classification
• Focal
– Usually hyaline-vascular
– Asymptomatic or symptoms due to mass effect
– Good prognosis with resection
• Multifocal
– Usually plasma cell or mixed
– Constitutional symptoms (fever, weight loss, night sweats)
– Chemotherapy, radiation
– Poorer prognosis
• HIV-associated
– Usually multifocal
– HHV-8 association
– High rate progression to lymphoma
– Also associated with Kaposi sarcoma
– Poor prognosis
Imaging
• Localized Castleman disease (hyaline-vascular)
– Large solitary mass
– May be locally invasive
– Heterogeneous soft tissue attenuation
– Intense contrast enhancement, large feeding vessels
– Flow voids on MRI
– FDG-avid on PET imaging
• Multifocal Castleman disease (plasma cell, mixed)
– Diffuse lymphadenopathy
– Less intense enhancement
– FDG-avid on PET imaging
Lung parenchymal Castleman disease
• Rare
• Usually in setting of multifocal disease
• Histopathology: lymphoid interstitial pneumonitis
• CT
– Centrilobular nodules
– Septal thickening
– Thin-walled cysts
There are two major histopathologic variants of Castleman disease: hyaline-vascular and plasma cell. The hyaline-vascular type is the most common (80–90%) and shows a follicular structure with tumor nodules composed predominantly of small lymphocytes and with large numbers of blood vessels in the interfollicular areas. The plasma cell type (10%) shows sheets of interfollicular cells and fewer blood vessels. 290 Mixed hyaline-vascular and plasma cell types can also occur, particularly in patients with multifocal disease.287.288. and 289. Furthermore, hyaline-vascular and plasma cell lesions can occur concomitantly at separate sites. 285
The pathogenesis of Castleman disease remains a subject of investigation. Although clonal expansion of certain lymphocyte populations is seen in some cases, Castleman disease is generally regarded as a reactive process that can eventually lead to lymphoma, particularly in patients with multifocal disease. There is evidence that some cases may be mediated by abnormal production of B lymphocyte growth factors, such as interleukin 6, leading to abnormal lymphoid proliferation. 285 There is also an association with infection by human herpes-8 virus (HHV-8), also known as the Kaposi sarcoma associated virus, particularly in HIV-positive individuals.291. and 292.
Castleman disease is currently best classified as unifocal, multifocal or HIV-associated disease.287.288. and 289. Patients with unifocal Castleman disease typically present in the fourth decade of life with a mass detected either incidentally or by virtue of symptoms related to mass-effect on neighboring mediastinal structures.276. and 286. Systemic symptoms, laboratory abnormalities, peripheral lymphadenopathy, or organomegaly are rare. About 90% of cases of unifocal Castleman disease are of the hyaline vascular variant. Unifocal disease is usually resected in toto and patients typically have a benign course thereafter.
Patients with multifocal Castleman disease typically present in the sixth decade of life with diffuse lymphadenopathy and systemic complaints such as fever, night sweats, and weight loss.287.288.289.293. and 294. Organomegaly, peripheral neuropathy, anemia, gamma globulin abnormalities, and elevated lactic dehydrogenase are common. 290 About 80% of cases of multifocal Castleman disease are of the plasma cell or mixed variants. Some patients with multifocal Castleman disease are infected with HHV-8, an association that increases in the HIV-positive population.287.288. and 289. Affected patients are usually treated with chemotherapy or radiation therapy with mixed results. Disease usually pursues an aggressive course, and development of frank lymphoma can occur.
Castleman disease that develops in HIV-positive individuals is almost always multifocal and pursues a very aggressive course. 295 There is a strong association with HHV-8 viral infection, and affected individuals may also develop Kaposi sarcoma.287.288.289.296.297. and 298. HIV-positive patients with multifocal Castleman disease have a very high risk of developing lymphoma as well. Prognosis is poor.
On chest radiographs, localized Castleman disease usually manifests as a focal, well-defined, smooth or lobular mediastinal or hilar mass (Fig. 14.43). The mass is typically quite large and most commonly occurs in the middle mediastinum or hila.276.299. and 300. Occasionally, the lesion is found in other sites, including the posterior mediastinum and chest wall.276.286. and 300. On noncontrast-enhanced CT, the mass is usually homogeneous and of soft tissue attenuation (Fig. 14.44). Calcification is uncommon (5–10%) and, when it occurs, is typically coarse and central in location (Fig. 14.45). Multifocal Castleman disease usually manifests on chest radiographs with diffuse mediastinal widening. On CT or MRI, multifocal lymph node enlargement is noted.
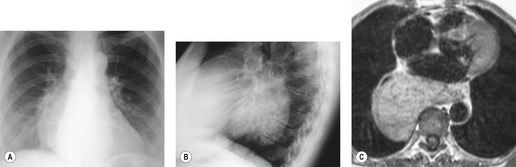 |
| Fig. 14.43 (With permission from McAdams HP, Rosado-de-Christenson M, Fishback NF, et al. Castleman disease of the thorax: radiologic features with clinical and histopathologic correlation. Radiology 1998;209:221–228.) |
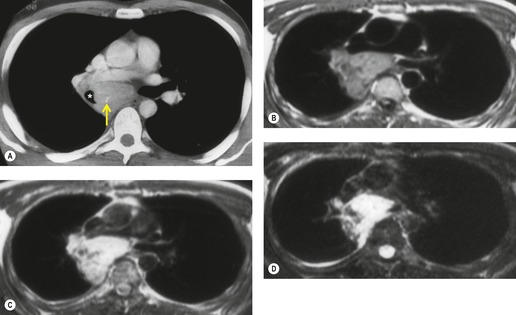 |
| Fig. 14.45 (With permission from McAdams HP, Rosado-de-Christenson M, Fishback NF, et al. Castleman disease of the thorax: radiologic features with clinical and histopathologic correlation. Radiology 1998;209:221–228.) |
Because of its highly vascular nature, hyaline-vascular Castleman disease usually enhances intensely following administration of intravenous iodinated or gadolinium-based contrast material (Fig. 14.45)276.286.293.301.302.303.304.305. and 306. – a distinctive feature that helps differentiate Castleman disease from many other mediastinal lesions including lymphoma. The lesions are also found to be highly vascular at angiography.299.301. and 307. Plasma cell Castleman disease is less vascular, and typically shows less enhancement after contrast administration. 258
On MRI, hyaline-vascular Castleman disease lesions are typically heterogeneous and have increased signal intensity compared with skeletal muscle on T1-weighted sequences (Figs 14.43 and 14.45).307.308. and 309. They become markedly hyperintense on T2-weighted sequences (Fig. 14.45). Low signal intensity septa are occasionally visible within the lesions. In larger lesions, flow voids in and around the mass may be identified and are important clues to the hypervascular nature of the mass. Because the lesions are hypervascular, diffuse enhancement following administration of intravenous gadolinium is common (Fig. 14.45). The FDG-PET imaging findings of Castleman disease have been described only in small series or case reports.310.311.312. and 313. In these reports, the lesions are usually quite metabolically active, whether uni- or multifocal in nature (Fig. 14.46).
Castleman disease may, very rarely, involve the lung parenchyma (Fig. 14.44). Reported manifestations include a pulmonary mass,290. and 314. centrilobular nodules, 315 septal thickening, 315 and thin-walled cysts. 315 Most patients with diffuse lung involvement have multifocal disease and the lung findings are usually due to associated lymphoid interstitial pneumonitis. 315
Diagnosis of lymphadenopathy
Important clues to the presence and sometimes the cause of mediastinal or hilar lymph node disease include enlargement and abnormal density on chest radiographs, attenuation on CT, or signal intensity on MRI. It is important to remember, however, that lymph nodes of normal size and attenuation or signal intensity can harbor disease, including malignancy.
Lymph node calcification
Calcification of intrathoracic lymph nodes (Box 14.12) is a common sequela of certain infections, particularly tuberculosis and histoplasmosis (Fig. 14.47). It can also be seen in other benign conditions such as sarcoidosis, silicosis, coal worker’s pneumoconiosis, amyloidosis, and Castleman disease. Lymph node calcification is very rarely due to neoplastic disease. Lymph node metastases from calcifying primary malignancies such as osteosarcoma, chondrosarcoma, carcinoid tumors, and mucinous colorectal and ovarian carcinomas (Fig. 14.48) may calcify.316. and 317. Lymph node calcification can occur after treatment of mediastinal lymphoma (Fig. 14.49), but is quite rare prior to treatment.318. and 319. CT clearly demonstrates lymph node calcification to better advantage than chest radiographs. MRI is limited, however, in its ability to show calcification, a notable disadvantage. 320
Box 14.12
Benign disease
Malignant disease
• Treated lymphoma and other neoplasms* (calcification in untreated lymphoma almost never occurs)
• Metastases from primary tumor
Osteosarcoma
Chondrosarcoma
Mucinous adenocarcinoma
Various patterns of lymph node calcification can be seen. Coarse irregular clumplike calcification of a part of the node and homogeneous calcification of the entire node are the two most common patterns. Lymph node calcification due to tuberculosis tends to involve the entire node whereas calcification due to sarcoid tends to be more punctate. Further, calcified lymph nodes due to tuberculosis tend to involve the mediastinum asymmetrically (usually unilateral), corresponding to the drainage patterns of primary infection; whereas diffuse, bilateral nodal involvement is more common in sarcoidosis. 321 A strikingly foamy pattern of calcification is described in AIDS patients with disseminated Pneumocystis jirovecii infection322. and 323. and in patients with metastatic mucinous neoplasms. Thin peripheral calcification – so-called ‘eggshell calcification’ (Fig. 14.50) – is seen in patients with coal worker’s pneumoconiosis, silicosis, and sarcoidosis.321.324. and 325. In one series, eggshell calcification was seen on chest radiographs in 3% of coal workers with greater than 30 years’ experience. 325 Eggshell calcification is rarely seen in other conditions, but has been reported in patients with amyloidosis, histoplasmosis, blastomycosis, and treated lymphoma. 277
Low attenuation on CT
Areas of low CT attenuation within enlarged nodes, likely due to necrosis (Fig. 14.51), is seen in a variety of conditions, including tuberculosis,326. and 327. nontuberculous mycobacterial infection, metastatic disease, particularly testicular tumor,328. and 329. and lymphoma. In one series, 16 of 76 patients with Hodgkin disease had low-attenuation lymphadenopathy on CT at presentation. 330 Some nodes have a prominent fatty hilum on CT. This feature is associated with benign disease and is usually thought to be the result of previous inflammation. Conversely, obliteration of the fatty hilum has been associated with metastatic disease. 331 Low-attenuation or even fatty nodes have also been described in patients with Whipple disease. 283
Contrast enhancement on CT
Contrast enhancement in enlarged nodes, when moderate in degree, is nonspecific and seen in patients with tuberculosis,327. and 332. fungal infection, 326 sarcoidosis, and metastatic neoplasm. When enhancement is dramatic, however, it suggests metastatic neoplasm from a highly vascular primary tumor such as melanoma, renal or thyroid carcinoma, carcinoid tumor, or leiomyosarcoma. Castleman disease is a further, though rare, cause of markedly enhancing lymph nodes (see Fig. 14.44). Peripheral, or rim, enhancement and central necrosis are features of tuberculosis (see Fig. 14.38) 327 and can be of diagnostic value in situations where tuberculosis is likely and metastatic carcinoma is not. 332
Chest radiographic signs of mediastinal lymph node enlargement
How large a mediastinal or hilar node has to be in order to be detected on chest radiographs is not easily established. It is likely that this size threshold depends upon the location of the node in the mediastinum, the presence of other mediastinal abnormalities such as tortuous great vessel or lipomatosis, and radiographic technique. Most nodes with a short-axis diameter of 2 cm or greater in the right paratracheal region, the aortopulmonary window, the hilar or the paravertebral regions should be detected on appropriately exposed posteroanterior chest radiographs. However, nodes in the pretracheal, left paratracheal, subcarinal, and paracardiac regions may be considerably larger than 2 cm in diameter and yet not be identified on chest radiographs.243. and 333.
Upper right paratracheal node enlargement (Fig. 14.52) (station 2R of the AJCC/UICC nomenclature) 334 (see p 69) manifests as uniform or lobular widening of the right paratracheal stripe. 335 When the paratracheal nodes become substantially enlarged, the lateral border of the superior vena cava may become convex rather than flat or concave. The apparent density of the superior vena cava may be increased and equal that of the aortic arch (normally the density of the superior vena cava in the right paratracheal area is less than that of the aortic arch). When the lower right paratracheal (azygos) nodes (station 4R) enlarge, they displace the azygos vein laterally so that the diameter of the combined shadow of the azygos node and vein enlarges (the normal diameter on an upright chest film should be 7 mm or less). 336
Enlargement of the upper left paratracheal nodes (station 2L) is frequently obscured by the shadows of the left carotid and subclavian arteries. These nodes have to be quite large to be detected on chest radiographs. If the aortopulmonary nodes (station 5) are substantially enlarged, they project beyond the aortopulmonary window and appear as a mass in the angle between the aortic arch and the main pulmonary artery (Fig. 14.53). 337 Enlargement of the anterior mediastinal nodes, i.e. nodes anterior to the aorta (station 6), the brachiocephalic artery (station 3), and the trachea (stations 2 and 4), must be substantial to be recognizable on chest radiographs. The resulting mediastinal abnormality is frequently bilateral and lobulated in contour (Fig. 14.54). Sometimes, the only finding of lymph node enlargement in these areas is increased opacity of the retrosternal area on the lateral view. Enlargement of the anterior intercostal, or internal mammary, nodes is best recognized on the lateral chest radiograph as well-marginated, retrosternal soft tissue masses along the course of the internal mammary arteries. Significant enlargement may result in upper parasternal opacities on the frontal radiograph. 338
The most useful sign of subcarinal node (station 7) enlargement on chest radiographs is a change from the normally concave contour of the superior portion of the azygoesophageal interface (Fig. 14.55) into a convex bulge. 339 Alteration of the contour of the azygoesophageal interface is, unfortunately, a relatively insensitive sign of subcarinal lymphadenopathy. It was noted in only 23% of the patients with subcarinal lymph node enlargement reported by Müller et al. 333 Effacement of the interface, without a focal bulge, can be seen with smaller volume nodes, but is a less specific finding for subcarinal pathology, particularly in children.340. and 341. Other chest radiographic findings of subcarinal node enlargement include increased opacity in the subcarinal region342 and obscuration of the medial margin of the bronchus intermedius. 333 Since the esophagus passes immediately behind the carina, subcarinal node enlargement may cause posterior displacement of the esophagus.
Enlarged paraesophageal nodes (station 8) and posterior mediastinal nodes result in displacement of the azygoesophageal and paraspinal interfaces.
CT of mediastinal lymph node enlargement
CT more readily demonstrates lymph node enlargement than chest radiography. The optimal CT technique for detection of enlarged mediastinal lymph nodes remains a subject of debate and is related to the rapidly changing nature of CT technology. In general, the thinner the slice collimation used, the better individual nodes will be shown. Thin-collimation images obtained on a multidetector spiral CT scanner will likely show more nodes than relatively thick-collimation images on a single-slice spiral or non-spiral scanner. However, whether this improvement in node detection makes a significant clinical difference to the patient is less certain and depends upon the specific clinical circumstances. Furthermore, although CT images are traditionally reviewed in axial format, several studies have shown that coronal reformat images from multidetector CT datasets obtained using thin slice collimation (0.5 mm or 2 mm collimation) performed better for the detection of enlarged lymph nodes than conventional 5 mm postcontrast scans.343. and 344. Again, whether this improvement translates into improved patient outcome is uncertain.
Whether or not intravenous contrast is necessary for detection of mediastinal lymphadenopathy also remains a subject of debate and is, for the most part, a decision that rests upon the experience of the interpreter and individual preference. One group compared contrast-enhanced with noncontrast CT for staging lung cancer and found that contrast-enhanced CT revealed more enlarged lymph nodes, particularly at the 2R station. 345 However, they also reported excellent overall agreement between the two studies for the presence and total number of enlarged lymph nodes, suggesting that the observed differences might not be clinically relevant. Another study compared contrast-enhanced with noncontrast CT for staging lung cancer and found that contrast-enhanced CT rarely changed the tumor stage determined by noncontrast CT. 346 These authors concluded that intravenous contrast was not needed for routine CT for lung cancer staging. A more recent study compared noncontrast with contrast-enhanced FDG-PET-CT for lung cancer staging, and found no difference for assessment of nodal metastases. 347
CT findings of mediastinal lymphadenopathy (Fig. 14.50, Fig. 14.51, Fig. 14.52, Fig. 14.53, Fig. 14.54, Fig. 14.55 and Fig. 14.56) include: an increase in size of individual nodes; focal mediastinal contour abnormalities or lobulations of the interface between the mediastinum and lung; invasion of surrounding mediastinal fat; coalescence of adjacent and enlarged nodes to form larger masses; and diffuse soft tissue attenuation throughout the mediastinum obliterating the mediastinal fat. Individually enlarged nodes are seen as round or oval soft tissue lesions in the mediastinum. Distinguishing enlarged nodes from normal vascular structures requires thorough knowledge of the normal arrangement of blood vessels and an understanding of the various anomalies and variations in the arrangement of the mediastinal vessels. Intravenous contrast enhancement may be needed to help distinguish vessels from lymph nodes in problematic cases.
Many authors have studied CT scans in normal patients in order to establish normal size criteria for mediastinal or hilar lymph nodes (see p 68).348.349.350. and 351. These studies suggest that the upper limit of normal for short-axis lymph node diameter is about 1 cm. Short-axis diameter is considered the standard for diagnosis because it is the most reproducible measurement and has the best correlation with lymph node volume at autopsy. 352 These studies must be interpreted with caution, however, as the importance of both ‘enlarged’ and ‘nonenlarged’ nodes varies depending upon node location, the clinical scenario, and the presence or absence of known or suspected malignancy. For example, nodes between 10 mm and 15 mm in short-axis diameter may be normally found in certain areas, such as the subcarinal region. Furthermore, nodes considerably smaller than 10 mm can harbor metastatic disease in patients with lung cancer. 353 Use of size alone to predict presence or absence of nodal malignancy is fraught with error, particularly in patients with lung cancer. 354
It is easier to identify and measure right-sided mediastinal lymph nodes than to evaluate the left-sided nodes, because of the more abundant mediastinal fat and less complex vascular anatomy on the right. 352 Subcarinal lymph node enlargement may be difficult to recognize at noncontrast CT. Important signs include: a soft tissue mass between the esophagus and either the left atrium or the intramediastinal portions of the right or left pulmonary arteries; or a soft tissue mass that extends into the azygoesophageal recess, posterior to the bronchus intermedius and the left lower lobe bronchus.
MRI of mediastinal lymph node enlargement
For the most part, MRI and CT provide comparable information regarding enlarged mediastinal nodes. However, MRI is frequently more time-consuming, more difficult for patients, and more limited in availability than CT at many centers. It can also be difficult to distinguish a cluster of small normal nodes from a single enlarged node or to detect intranodal calcification at MRI. 320 Furthermore, differences in signal intensity at spin-echo MRI have not proved to be a reliable method for differentiating benign from malignant lymphadenopathy. For these reasons, MRI is currently used primarily for patients for whom the use of iodinated intravenous contrast material is contraindicated.247. and 355.
There has been considerable recent interest, however, in developing new MR techniques for imaging nodal metastases from a variety of malignancies including lung cancer. One group reported increased accuracy for differentiating benign from malignant nodes at 3.0 T field strength. 331 Others have reported positive results with contrast agents such as gadolinium-DTPA356. and 357. or molecular imaging techniques such as small superparamagnetic iron oxide (USPIO) particles.358.359. and 360. In a recent series, USPIO-enhanced MRI was shown to be more accurate that FDG-PET for detection of nodal metastases in a rabbit model. 360 Furthermore, new pulse sequences such as diffusion-weighted MRI are being investigated and have also been recently shown to be more accurate than FDG-PET for mediastinal nodal staging in patients with lung cancer. 361 While these results are promising, their impact and eventual role in staging patients with suspected mediastinal nodal metastases remains to be seen.
PET imaging of mediastinal lymph node enlargement
PET imaging, using a variety of positron-emitting agents, most commonly FDG, has assumed a dominant role in the assessment of mediastinal lymph nodes, especially in patients with known or suspected malignancy. The role and influence of FDG-PET imaging in this regard very much depends upon the clinical scenario and source of primary malignancy and is considered in more detail in sections dealing with specific conditions. However, a few caveats are appropriate. First, while FDG-PET greatly improves the accuracy of assessment of suspected mediastinal nodal metastases, it is not perfect; false positives and negatives occur and are not infrequent. 354 Second, a variety of benign causes of lymph node enlargement also show increased metabolic activity on FDG-PET imaging, including sarcoidosis, tuberculosis, and histoplasmosis (see Fig. 14.40).242.362. and 363. Third, combined PET-CT is probably more accurate for assessment of mediastinal lymph nodes than either CT or PET alone, especially in patients with lung cancer.363.364. and 365.
Chest radiographic findings of hilar lymph node enlargement
The findings of hilar node enlargement on chest radiographs are enlargement of the hilum, increased lobulation of the hilar contours, a rounded mass in a portion of the hilum that does not contain major vessels (Fig. 14.56; see also Figs 14.37 and 14.39), and increased density of the hilum. 366 In certain highly vascular regions of the hila (e.g. the intersection of the right superior pulmonary vein and interlobar artery and the inferior aspect of the hilum where the inferior pulmonary veins intersect the lower lobe segmental arteries), nodal enlargement must be substantial to be recognized radiographically. Enlarged nodes adjacent to the lower lobe arteries increase the overall diameter of the hilum (the transverse diameter of each lower lobe artery should be no greater than 16 mm) and result in a lobular rather than the normal tubular configuration. Lymph node enlargement in the upper portions of the hila is often easily detected because the vessels in these regions are normally small. Even mild nodal enlargement can be recognized on the lateral view when the enlarged nodes lie posterior to the right main bronchus and bronchus intermedius (Fig. 14.57), because lung normally contacts the posterior wall of the airway in this region. Another important region to evaluate for hilar lymphadenopathy on the lateral view is the angle formed by the lower lobe bronchi and the middle lobe or lingular bronchus (Fig. 14.58) – the so-called inferior hilar window. 367
It may, at times, be very difficult to distinguish enlarged hilar lymph nodes from enlargement of the hilar arteries due to pulmonary hypertension (Fig. 14.59). Correct diagnosis depends on determining that the abnormality is truly centered on the pulmonary arteries and on evaluating the degree of lobulation. A hilar mass in a location that is normally devoid of vessels clearly favors nodal enlargement. Enlarged central pulmonary arteries usually retain their tubular configuration. Lobulated hilar enlargement favors lymphadenopathy. If needed, contrast-enhanced CT will answer the question.
CT of hilar node enlargement
The recognition of hilar node enlargement at CT is facilitated by intravenous contrast opacification of the hilar vessels (see Fig. 14.37). 368 Lymph nodes generally do not enhance to the same degree as blood vessels. The majority of nodes in the hilum, except those around the lower hilum, are normally less than 3 mm in short-axis diameter. 369 Nonenhancing hilar tissue that is up to 7 mm in short-axis diameter is commonly seen in the lower hila, however. 369 If the examination is performed without contrast, then the recognition of nodal enlargement depends on demonstrating rounded soft tissue densities that are too large to be blood vessels (see Figs 14.56 and 14.58).370.371.372. and 373. The smallest node that can be reliably detected on noncontrast CT varies with location. Some portions of the hilum are normally devoid of vessels greater than 5 mm in diameter and thus relatively small nodes may be detected as contour abnormalities in these regions. In other regions of the hila, however, the vessel diameters may be 15 mm, or greater if there is increased pulmonary blood flow or pulmonary arterial hypertension, limiting detection of all but the largest nodes. Perhaps the most sensitive area for detection of hilar lymphadenopathy on noncontrast CT is the region immediately behind the right main bronchus and its divisions – the right upper lobe bronchus and the bronchus intermedius – because, in these regions, the lung normally contacts the posterior wall of the airway. 374 Increased soft tissue opacity, especially if it is lobulated, in this region is suggestive of lymphadenopathy. The equivalent area in the left hilum is occupied by the descending aorta and left descending pulmonary artery; therefore, only a small portion of lung contacts the posterior wall of the left main bronchus, 375 limiting detection of lymphadenopathy in this region. The most difficult, and therefore the least sensitive, area for detection of hilar lymphadenopathy is the central portion of the right hilum, where the right superior pulmonary vein crosses directly anterior to the right pulmonary artery and its major divisions. Additionally, there are fat pads at the bifurcation of the right pulmonary artery that can resemble lymph node enlargement on axial CT (Fig. 14.60). It can be difficult to recognize the fatty nature of these pads because of partial volume averaging with the adjacent arteries and lung. 376
MRI of hilar node enlargement
As is the case with imaging mediastinal lymph node enlargement, MRI and CT provides comparable information regarding enlarged hilar nodes. However, hilar node enlargement may be more easily recognized with MRI than with noncontrast CT.320.326.377. and 378. For the most part, MRI is used primarily in patients for whom the use of iodinated intravenous contrast material is contraindicated.247. and 355.
Pitfalls in the diagnosis of intrathoracic lymph node enlargement
A variety of anatomic structures can be confused with mediastinal lymph node enlargement,379.380. and 381. including enlarged blood vessels and vascular variants such as aortic anomalies (p 979), azygos continuation of the inferior vena cava, varices of the azygos or pulmonary vein,382.383.384. and 385. and aneurysmal dilatation of the brachiocephalic vein or superior vena cava.385.386. and 387. Fluid in the various pericardial recesses can occasionally mimic lymphadenopathy or other mediastinal masses.388. and 389. Choi et al. 388 described a series of patients with posterior superior pericardial recesses that extended cephalad to the level of the aortic arch (‘high-riding’) and were misdiagnosed as lymphadenopathy. Some of these pitfalls are illustrated in Fig. 14.61, Fig. 14.62 and Fig. 14.63.
Lymphovascular tumors of the mediastinum
Blood vessel tumors
Blood vessel tumors in the mediastinum are rare and frequently benign. Capillary or cavernous hemangiomas390 are the most common lesions. Hemangioendothelioma, 391 hemangiosarcoma, hemangiopericytoma, 392 hemangioendothelioma, 393 and mixed lymphatic and blood vessel lesions, such as lymphangiohemangiomas,394.395. and 396. are very rare.
Hemangiomas are rare mediastinal tumors that account for less than 0.5% of all mediastinal masses. 397 Mediastinal hemangiomas usually occur in the anterior (68%) or posterior mediastinum (22%), although multicompartment involvement is found in up to 14% of cases.398.399.400. and 401. Most mediastinal lesions are cavernous hemangiomas and are composed of large interconnecting vascular spaces with varying amounts of interposed stromal elements such as fat and fibrous tissue. Focal areas of organized thrombus can calcify as phleboliths. Affected patients are usually asymptomatic.
On chest radiographs, hemangiomas manifest as sharp, well-marginated mediastinal masses (Fig. 14.64). Phleboliths are seen in less than 10% of cases but, when present, are diagnostic. CT typically reveals a heterogeneous mass with intense central and peripheral rim-like enhancement after administration of intravenous contrast.391.397.398.402. and 403. Hemangiomas typically have heterogeneous signal intensity on T1-weighted images. In lesions with significant stromal fat, linear areas of increased signal intensity on T1-weighted images can occasionally be identified. The central vascular spaces typically become markedly hyperintense on T2-weighted images, a suggestive feature (Fig. 14.64). 404
Mixed lymphangioma/hemangioma is a variant of hemangioma seen most frequently in children and young adults. 405 The lesion may be localized to the thorax or occur as a more systemic process. When it occurs in the chest, the lesion may involve the mediastinum, pleura, and chest wall as a single process causing widespread lobular soft tissue swelling, bone destruction, and chylous pleural effusion. This destructive form of the disease is known as Gorham disease.406. and 407. Cystic angiomatosis is another, probably distinct form of widespread lymphangiomatosis408 in which lymphangiomas and hemangiomas may coexist. Many different sites in the body are involved, including the mediastinum, pericardium, and pleura. Multiple lytic lesions may be seen in the bones. 409 The condition is most frequently seen in children and young adults. Lymphangiomatosis is discussed further in Chapter 16.
Mediastinal hemorrhage
Trauma to the aorta, branch vessels, or spine is a frequent cause of mediastinal hemorrhage (see p 1121, Chapter 17). Common causes of spontaneous mediastinal hemorrhage include aortic dissection (see p 967), rupture of an aneurysm, misplacement of central catheters (Fig. 14.65), bleeding disorders, or anticoagulant therapy. Other less common causes of mediastinal hemorrhage include chronic hemodialysis, 410 bleeding into preexisting mediastinal tumors, such as thymic masses and thyroid goiter, radiation vasculitis,411. and 412. and severe vomiting.413. and 414.
Patients with mediastinal hemorrhage can be asymptomatic or present with substernal chest pain that may radiate to the back. Its investigation depends on the probable cause. CT is usually the first line of investigation after chest radiography to confirm the presence of hemorrhage and occasionally to elucidate its cause. The chest radiographic findings of mediastinal hemorrhage depend upon the cause and source of the bleeding. Blood may affect one mediastinal compartment and manifest as a focal mass (Fig. 14.65), or may dissect freely throughout the mediastinum and manifest as diffuse mediastinal widening. 415 Blood may also dissect extrapleurally over the lung apices, giving rise to the important sign of apical capping. 416 When the hemorrhage is severe, blood may rupture into the pleural cavity or dissect into the lung along perivascular and peribronchial sheaths, resulting in opacities that resemble pulmonary edema. 417
The appearance of mediastinal hemorrhage on CT can be fairly characteristic (Fig. 14.65). Linear bands of soft tissue attenuation are seen interspersed with mediastinal fat in affected regions of the mediastinum. On occasion, it is possible to appreciate the high attenuation values of fresh thrombus on noncontrast CT images. A focal hematoma may, however, be difficult to distinguish from a solid mediastinal mass on CT. The appearance of hemorrhage on MRI varies with the age of the hemorrhage (Fig. 14.66). In the hyperacute phase, there is low signal on T1-weighted and high signal on T2-weighted images. Over the ensuing days, the signal on the T1-weighted images rises and there is a period during the subacute phase in which high signal is seen on both T1- and T2-weighted images. Thereafter, complex signal patterns are seen in which the signal intensity depends on the amount of water in the area of hemorrhage and the degree of conversion from methemoglobin to ferritin and hemosiderin. 418
Mediastinitis
Acute mediastinitis
Acute mediastinitis is a potentially life-threatening, but fortunately rare, condition that requires prompt diagnosis and treatment. Spontaneous or iatrogenic esophageal rupture is the most common cause, accounting for up to 90% of cases.419. and 420. Other causes of acute mediastinitis include necrosis of neoplasm, extension of infection from the neck, pharynx, teeth,421.422.423.424. and 425. retroperitoneum, lungs, pleura, or adjacent bones and joints, 426 and mediastinitis after cardiac surgery. 427 Acute mediastinitis may also be associated with empyema or subphrenic abscess. Clinically, affected patients are often very ill with chills, high fever, tachycardia, and chest pain. Circulatory shock is frequent. Dysphagia is common in those patients in whom the mediastinitis is caused by perforation of the esophagus. Diffuse mediastinitis has a particularly high mortality.
Esophageal perforation is usually caused by penetrating trauma, particularly from surgery, endoscopy, or swallowing sharp objects such as chicken bones. In young children, the possibility of child abuse as a cause of pharyngeal or esophageal perforation must be considered. 428 Spontaneous perforation may occur, as in the Boerhaave syndrome, when forceful vomiting causes a tear in the esophageal wall. The tear in the esophagus is usually just above the gastroesophageal junction. It may be of any depth, but is usually confined to the mucosa, in which case bleeding may occur but there is no immediate danger of mediastinitis. If the tear is complete, however, air, alimentary juices, and food leak into the mediastinum resulting in mediastinitis.
The primary imaging features of acute mediastinitis are mediastinal widening, pneumomediastinum, obliteration of fat planes, localized fluid collections, and abscess formation (Figs 14.67 and 14.68). 429 Mediastinal widening is the result of inflammatory swelling or abscess formation within the mediastinum. Because so many cases of acute mediastinitis are secondary to esophageal perforation, an important clue to the diagnosis is air within the mediastinum, a feature that may be difficult to appreciate on chest radiographs. The air may be bubbly or streaky and may be localized or widespread in distribution. As with all types of pneumomediastinum, the air may extend into the neck or retroperitoneum. Accompanying pleural effusions in one or both pleural cavities are also common. Pleural effusion tends to be right sided in patients with iatrogenic, mid-esophageal perforation and left sided in patients with spontaneous, distal esophageal perforation (Boerhaave syndrome). In patients with Boerhaave syndrome, the effusion is particularly striking on the left and is often accompanied by consolidation of the left lower lobe (Fig. 14.67). 430 All these features are better demonstrated on CT than on chest radiographs.429.431. and 432. Findings of esophageal perforation on CT include periesophageal fluid collections (100%), extra-luminal mediastinal air (100%), esophageal wall thickening (82%), and pleural effusion (82%). 433 The site of perforation is rarely identifiable on CT (18%). 433 Contrast esophagography can be critical in determining the presence and precise location of esophageal perforation. 430 In cases of acute mediastinitis without discrete abscess formation, CT may show diffuse obliteration of normal fat planes, and gas bubbles may be scattered throughout the mediastinum (Fig. 14.69). When a walled-off abscess develops, the gas may be seen in discrete rounded collections or as air–fluid levels (Fig. 14.70). Mediastinal abscesses may be solitary, but are frequently multiple. CT is an invaluable guide should percutaneous drainage be indicated.429. and 434. CT may also show important associated abnormalities such as jugular vein thrombosis, pericardial effusion, or rupture of the hypopharynx or esophagus.
Descending cervical mediastinitis is an uncommon, but potentially life-threatening, cause of mediastinitis.423.424.425. and 435. These infections begin in the head and neck region and spread via fascial planes (usually in the prevertebral space) into the middle and posterior mediastinum. Typical causes include odontogenic infection, suppurative tonsillitis, and retropharyngeal abscess. CT in cases of descending cervical mediastinitis shows fluid collections in the mediastinum that may be contiguous with a fluid collection in the cervical region. 435 CT is essential for confirming the diagnosis, assisting in fluid aspiration to confirm infection, and for monitoring response to therapy.
Mediastinitis after cardiac surgery is uncommon, occurring in only 0.5–3% of patients.427.429.436.437.438.439. and 440. Mediastinitis often occurs in the setting of sternal dehiscence. Radiographic features of sternal dehiscence include the midsternal stripe and the wandering wire signs (Fig. 14.71). A very thin vertical lucency (midsternal stripe) in the upper third of the sternum can often be seen on well-centered radiographs of patients after uncomplicated sternotomy. This lucent line should not exceed 2 mm in thickness, nor extend below the first sternal wire. Progressive mid-sternal lucency or a mid-sternal stripe greater than 3 mm in thickness after sternotomy suggests possible dehiscence. 441 Changes in position or orientation of sternal wires (wandering wire sign) also suggest dehiscence. 442 However, neither sign is predictive of mediastinitis complicating dehiscence. 443
CT is therefore frequently performed in patients with clinically suspected mediastinitis but can be difficult to interpret since fluid and air collections, hematomas, pleural effusions, and increased attenuation of anterior mediastinal fat, all potential findings of mediastinitis, are common expected findings in the immediate postoperative period (Fig. 14.72). These findings generally resolve, however, in the first days and weeks after median sternotomy. One study found that mediastinal air or fluid collections seen on CT in the first 2 weeks after sternotomy were not specific for mediastinitis. 437 However, such findings were highly indicative of mediastinitis after 2 weeks. Air or fluid collections that appear de novo or that progressively increase without other explanation are also suggestive of mediastinitis (Figs 14.70 and 14.73). 444 Needle aspiration of fluid collections may be necessary to rule out infection when mediastinitis is suspected (Fig. 14.72). CT is most useful for distinguishing patients with substantial retrosternal fluid collections that require open drainage from those that have only superficial wound infections. CT has limited ability for detecting early changes of sternal osteomyelitis. As noted above, minor degrees of sternal separation are common in asymptomatic patients after uncomplicated operations.436. and 438. However, gross sternal destruction, indicative of osteomyelitis, is occasionally seen on CT (Fig. 14.73). Radiolabeled leukocyte scanning (indium-111, 99mTc-hexamethylpropylene amine oxime [HMPAO]) can also be used to help diagnose sternal osteomyelitis in the setting of mediastinitis.445. and 446.
Fibrosing mediastinitis
Fibrosing mediastinitis (sclerosing mediastinitis or mediastinal fibrosis; Box 14.13) is a rare disorder caused by proliferation of acellular collagen and fibrous tissue within the mediastinum. 447 It is thought that most cases in the USA are caused by an abnormal immunologic response to Histoplasma capsulatum antigens in genetically susceptible individuals.448.449. and 450. However, fibrosing mediastinitis is rare, even in areas where histoplasmosis is endemic, and recovery of organisms in affected specimens is unusual. Other etiologies that are likely more important in other parts of the world where H. capsulatum is rare include M. tuberculosis, autoimmune disease, Behçet disease, radiation therapy, trauma, and drugs such as methysergide.451.452. and 453. Importantly, an idiopathic form of fibrosing mediastinitis is also now recognized.454. and 455. A rare familial form associated with retroperitoneal fibrosis, sclerosing cholangitis, Riedel thyroiditis, and pseudotumor of the orbit has also been reported. 456
Box 14.13
Etiology
• Infection
– H. capsulatum (in USA)
– Other fungi
– M. tuberculosis
• Radiation, autoimmune disease, drug therapy
• Idiopathic
Clinical – symptoms related to mediastinal structures involved
• Airway
– Recurrent pneumonia
– Persistent atelectasis
– Hemoptysis
• Pulmonary vein
– Mimics mitral stenosis
– Dyspnea, hemoptysis
• Superior vena cava
– Distended neck veins
– Facial swelling and edema
Imaging
• When related to infection
– Focal invasive mediastinal mass
– Typically unilateral
– Calcification in most
• When idiopathic
– Focal or diffuse mediastinal soft tissue mass
– Calcification uncommon
• Caveats
– Chest radiograph frequently underestimates extent of mediastinal disease
– CT best for showing calcification, mediastinal invasion
– CT angiography/MRI good for showing vascular involvement
• Differential diagnosis
– Lymphoma
– Calcifying mediastinal metastases
Fibrosing mediastinitis is characterized by progressive proliferation of fibrous tissue within the mediastinum that encases and eventually obstructs vital structures such as the vena cava, the pulmonary arteries and veins, and the airways. Fibrosis due to histoplasmosis is often focal in nature. In older reports, idiopathic disease was described as more diffuse in distribution. 457 However, more recent reports describe more focal mediastinal involvement in cases of idiopathic fibrosis.454. and 455.
In areas where histoplasmosis is endemic, affected patients usually present in the second through fifth decades of life with signs and symptoms of cough, recurrent pulmonary infection, hemoptysis, or chest pain. 449 Pulmonary venous obstruction may result in symptoms that mimic mitral stenosis. Patients with superior vena cava obstruction may present with swelling of the face and distension of the neck veins. 458 In other parts of the world where histoplasmosis is unusual, the age range at presentation is much broader. 451
Chest radiographs frequently underestimate the extent of mediastinal disease (Fig. 14.74) and can be normal.459.460.461.462.463.464.465.466. and 467. When abnormal, chest radiographic findings vary somewhat depending on the etiology of fibrosis and the site and nature of the mediastinal abnormality. Disease due to infection, usually histoplasmosis, typically results in focal or diffuse calcified mediastinal masses that may be evident radiographically (Fig. 14.75). Idiopathic fibrosis results in either a focal454. and 455. or diffuse mediastinal abnormality without evident calcification (Fig. 14.76). 468 In either setting, the secondary effects of mediastinal fibrosis may also be evident on chest radiographs. These include findings of airway narrowing, parenchymal consolidation or atelectasis due to airway obstruction, oligemia due to pulmonary artery obstruction, or septal thickening and pleural effusion due to pulmonary venous obstruction (Fig. 14.77).
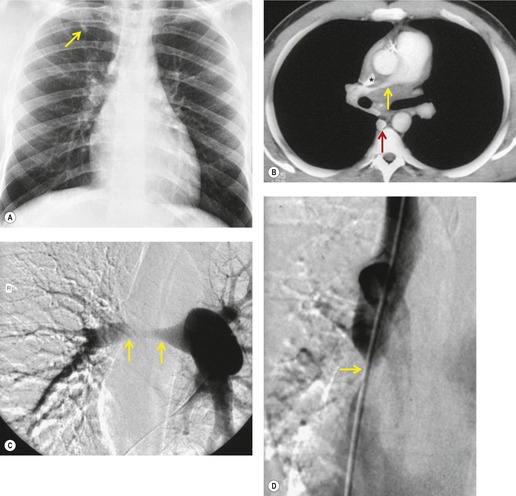 |
| Fig. 14.74 (With permission from Rossi SE, McAdams HP, Rosado-de-Christenson ML, et al. Fibrosing mediastinitis. RadioGraphics 2001;21:737–757.) |
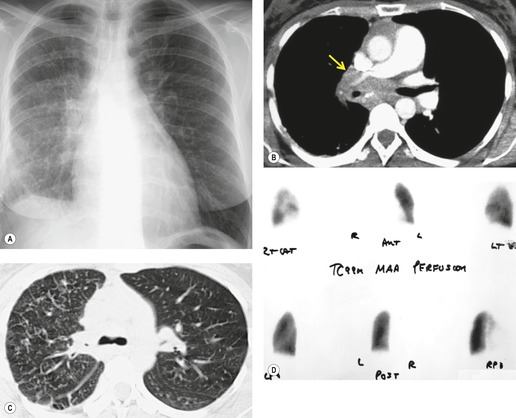 |
| Fig. 14.77 (With permission from Rossi SE, McAdams HP, Rosado-de-Christenson ML, et al. Fibrosing mediastinitis. RadioGraphics 2001;21:737–757.) |
CT or MRI are most useful for evaluation of fibrosing mediastinitis.259.449.451.465.469.470. and 471. When disease is due to histoplasmosis or tuberculosis, CT usually shows a focal, infiltrative mediastinal or hilar mass that is often extensively calcified (Figs 14.74, 14.75 and 14.77). When disease occurs idiopathically, CT may show either a focal masslike opacity454. and 455. or more diffuse encasement of mediastinal structures by soft tissue attenuation masses that obliterate normal fat planes (Fig. 14.76). Calcification is uncommon in lesions due to idiopathic fibrosis. In either setting, CT is also useful for demonstrating airway, pulmonary arterial and venous involvement.
On MRI, the process is typically of heterogeneous signal intensity on T1- and T2-weighted images. 447 Markedly decreased signal intensity on T2-weighted images is occasionally seen and is suggestive of the fibrotic or calcific nature of the process.472. and 473. MRI can demonstrate the infiltrative nature of the fibrosis and the narrowing of the major vessels and bronchi as well as, if not better than, CT.460.470.472.474. and 475. However, CT is better for demonstrating calcification within the lesion, a finding that is critical for differentiating fibrosing mediastinitis from other infiltrative disorders of the mediastinum such as metastatic carcinoma or lymphoma.
Radionuclide ventilation–perfusion scanning can be used to diagnose pulmonary arterial or airway obstruction (Fig. 14.77).473. and 476. Venography, pulmonary arteriography (Fig. 14.74), or CT angiography may show smooth, tapered narrowing of the superior vena cava and brachiocephalic veins, together with numerous dilated collateral veins, and may also show narrowing of the central pulmonary arteries.449. and 467. Lesions can be either metabolically active477. and 478. or negative479 on FDG-PET imaging. Barium swallow may show narrowing of the esophagus and, in rare instances, may show varices resulting from esophageal venous collaterals, so-called downhill varices.
The prognosis for affected patients is often unpredictable; disease may progress, remain stable for many years, or even spontaneously regress. 447 Mortality rates up to 30% are reported. 449 Patients with subcarinal or bilateral fibrosis may have a slightly higher mortality than patients with more localized disease. 449 Causes of death include recurrent pneumonia, hemoptysis, or cor pulmonale. 447 Because many cases in the USA are caused by an inflammatory reaction to H. capsulatum infection, some patients have been treated with systemic antifungal agents or corticosteroids, with variable success. 447 If disease is localized, surgical resection can be curative or result in symptomatic improvement. Bilateral mediastinal involvement may preclude surgery, however. Symptomatic patients may also be treated by percutaneous therapies directed at occluded or severely stenosed airways, pulmonary arteries, or vena cava.480. and 481. Laser therapy, balloon dilation, and intravascular or endobronchial stent placement have all been used with success to treat affected patients (Fig. 14.78). 447
Mediastinal panniculitis
Panniculitis is an inflammatory process of fat leading to focal fat necrosis. It is most commonly encountered in subcutaneous mesenteric fat. Mediastinal panniculitis is a very rare condition that is usually seen in patients with Weber–Christian disease, it may cause focal mediastinal widening, and may therefore be mistaken for neoplasm. CT in one case showed masslike accumulations of soft tissue (shown to be fibrosis and inflammation on histopathologic examination) interspersed with fat in the mediastinum. 482 On MRI, the mass was very heterogeneous. 482
Neurogenic tumors of the mediastinum
For purposes of discussion, the neurogenic tumors (Box 14.14, Box 14.15 and Box 14.16) can be classified as tumors of nerve sheath origin, those of ganglion cell origin, and tumors of the paraganglionic cells.483.484. and 485.
Box 14.14
• Nerve sheath tumors
– Schwannoma
– Neurofibroma
– Malignant tumor of nerve sheath origin
• Ganglion cell tumors
– Ganglioneuroma
– Ganglioneuroblastoma
– Neuroblastoma
• Paragangliomas
Box 14.15
Tumors of nerve sheath origin
• Chest radiography
– Round or oval
– Enlarged neural foramina
– Pressure erosion on bone
– Rarely calcify
• CT
– Neurofibroma – homogeneous soft tissue mass, can be near-water attenuation and mimic a cyst
– Schwannoma – usually more heterogeneous, calcification
– Both enhance heterogeneously
– Malignant nerve sheath tumor – heterogeneous, bone destruction, metastases
• MRI
– Intraspinal extension (‘dumbbell’ lesion) in 5%
– Neurofibroma – target lesion on T1, T2
Tumors of ganglion cell origin
• Chest radiography
– Elongated along axis of spine
– May also cause bone changes
– May calcify
• CT
– Ganglioneuroma – homogeneous soft tissue mass
– Neuroblastoma – heterogeneous, invasive
– Calcification is common
• MRI
– Intraspinal extension
Box 14.16
• Histopathology
– Arise from chromaffin cells
– Benign or malignant
• Terminology
– Within adrenal = pheochromocytoma
– Outside adrenal = paraganglioma
• Location (mediastinal)
– Two-thirds near aortic arch (aortic body tumors)
– One-third paravertebral
– Rarely within heart
• Mediastinal paragangliomas usually nonfunctional
• CT findings
– Homogeneous or heterogeneous, central necrosis
– Hyperenhancing
• MRI
– Isointense T1 signal
– Hyperintense T2 signal
– Intratumoral flow voids due to vessels
• FDG-PET findings
– Metabolically active
The nerve sheath tumors include schwannomas, neurofibromas, and malignant tumors of nerve sheath origin. They account for up to 65% of mediastinal neurogenic tumors. 486 Schwannomas are the most common intrathoracic nerve sheath tumors. 487 Neurofibromas, particularly when multiple, and malignant nerve sheath tumors are strongly associated with neurofibromatosis. Patients with neurofibromatosis may develop large plexiform masses of neurofibromatous tissue in the mediastinum (Fig. 14.79).488.489. and 490. Granular cell myoblastomas, which are believed to be of Schwann cell origin, are rarely found in the mediastinum.491. and 492.
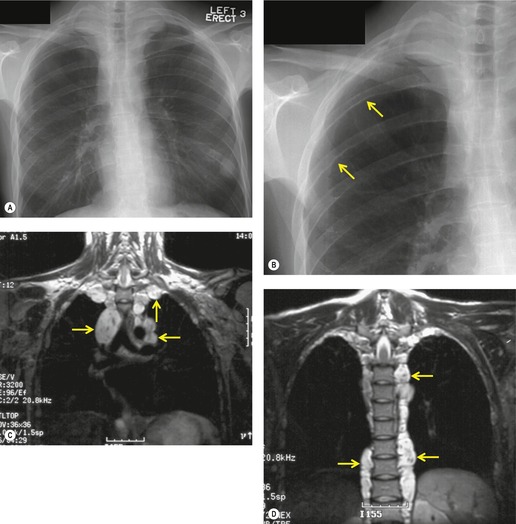 |
| Fig. 14.79 (With permission from Rossi SE, Erasmus JJ, McAdams HP, et al. Thoracic manifestations of neurofibromatosis-I. AJR Am J Roentgenol 1999;173:1631–1638.) |
Almost all intrathoracic nerve sheath tumors arise either from the intercostal (Fig. 14.80) or from sympathetic nerves, the rare exceptions being neurofibromas or schwannomas of the phrenic or vagus nerves. Many arise adjacent to the spine and, in about 5% of cases,14. and 493. extend through the neural foramina into the spinal canal (the so-called ‘dumbbell tumor’) (Fig. 14.81).
Most nerve sheath tumors of the mediastinum are benign. Affected patients are typically asymptomatic and the tumors are often discovered as incidental findings on chest imaging studies. In contrast to the ganglion cell tumors, nerve sheath tumors are rare in patients under the age of 20 and virtually nonexistent in patients who are less than 10 years old, except in patients with neurofibromatosis. Malignant tumors of nerve sheath origin are uncommon and almost always occur in patients with neurofibromatosis.483. and 485. Affected patients typically present with pain.
The tumors of ganglion cell origin comprise a spectrum from benign ganglioneuroma to malignant neuroblastoma; ganglioneuroblastoma is an intermediate form of low malignant potential. 494 Neuroblastoma and ganglioneuroblastoma may occasionally mature into the more benign form. 495 The mediastinum is the second most common primary site (after the adrenal gland) for tumors of ganglion cell origin. 496 Approximately one-third to one-half of mediastinal neuroblastomas arise primarily in the mediastinum.497. and 498. The remainder occur secondary to either lymph node metastases or intrathoracic spread from a tumor arising primarily in the adrenal gland.
Neuroblastoma and ganglioneuroblastoma are essentially tumors of childhood.497. and 499. Less than 10% occur in patients older than 20 years of age.487. and 500. In children less than 1 year of age, a neurogenic tumor is virtually certain to be one of these two types. 501 Ganglioneuroma has a broader and more even age distribution, ranging from 1 to 50 years.487. and 501. Urinary vanillylmandelic acid and homovanillylmandelic acid levels may be elevated in patients with neuroblastoma and ganglioneuroblastoma and are useful diagnostic markers. 502
Imaging of neurogenic tumors (Box 14.15)
Most neurogenic tumors manifest as well-defined masses with smooth or lobulated contours (Fig. 14.82, Fig. 14.83 and Fig. 14.84).487.500. and 503. When localized, it is not possible to distinguish benign from malignant lesions. The tumors may be almost any size and some are very large, occupying most of a hemithorax. Except for vagal and phrenic nerve tumors, and the occasional neuroblastoma, neurogenic tumors are typically situated in the posterior mediastinum501 or grow along intercostal nerves. Those that arise adjacent to the upper thoracic spine may occupy the lung apex and appear as a well-marginated apical mass (Fig. 14.84).
Most neurogenic tumors are spherical in nature, but some ganglion cell tumors are elongated along the spine, paralleling the vertical orientation of the sympathetic chain (Fig. 14.85). It may be possible to distinguish between a ganglion cell tumor and a nerve sheath tumor by observing: (1) the shape of the tumor mass, since the ganglion cell tumors are frequently elongated along the mediastinum with tapered superior and inferior margins, whereas nerve sheath tumors are more spherical in shape with more acute angles at their margins; and (2) that ganglion cell tumors arise slightly more anteriorly with their center alongside the vertebral body, whereas nerve sheath tumors are centered on the neural foramen, or are closely adherent to the chest wall.
Calcification can be seen in all types of neurogenic tumors (Figs 14.85 and 14.86). Approximately 10% of primary mediastinal neuroblastomas are visibly calcified on chest radiographs,487. and 497. a figure considerably lower than that reported for neuroblastomas arising in the abdomen. The frequency of calcification detectable at CT is substantially higher. In neuroblastoma, the calcification is usually finely stippled, whereas in ganglioneuroblastoma and ganglioneuroma (Fig. 14.85), it is denser and coarser, occurring most frequently in the larger, more benign lesions. Nerve sheath tumors rarely calcify.487. and 488. When present, calcification is typically curvilinear and peripheral in nature and is seen in only very large masses.
Because neurogenic tumors tend to arise adjacent to bone and grow slowly, they can cause pressure erosions of adjacent ribs and vertebrae (Figs 14.81, 14.83 and 14.85) – an important diagnostic feature. The bone in immediate contact with the tumor shows a scalloped edge; usually the bony cortex is preserved, and frequently it is thickened. The ribs may be thinned and splayed apart, and the intervertebral foramina may appear enlarged. With larger lesions, the absence of changes in the adjacent bones argues against the diagnosis of a neurogenic tumor. Bone changes are most frequently seen with the tumors of ganglion cell origin, perhaps because these tumors are frequently large at presentation and occur in pediatric patients with a rapidly growing skeleton. Large tumors may be associated with scoliosis. 488 Frank destruction of bone appears to be a sign of malignancy,487.488. and 504. as is associated pleural effusion. 501
On noncontrast CT, schwannomas are often of mixed attenuation, and may have regions that are close to water attenuation485.500.504.505.506. and 507. due either to hypocellularity or to cystic degeneration (Fig. 14.83).508. and 509. Neurofibromas tend to be more homogeneous on noncontrast CT.485. and 506. Nerve sheath tumors are typically vascular and enhance after administration of intravascular contrast. A variety of enhancement patterns have been described including homogeneous, diffuse heterogeneous (with cystlike regions of nonenhancement), rim enhancement, and central enhancement with a hypoattenuating rim.485. and 504. Malignant nerve sheath tumors are typically heterogeneous on both contrast-enhanced and noncontrast CT; local invasion and bone destruction as well as metastatic foci to the pleura or lungs suggest the diagnosis (Fig. 14.87).485. and 509. Ganglioneuromas usually manifest as homogeneous or heterogeneous masses of low-attenuation lesions on both contrast-enhanced and noncontrast CT (Fig. 14.85). 485 Neuroblastomas manifest as heterogeneous soft tissue masses that show extensive local invasion (Fig. 14.88). 485
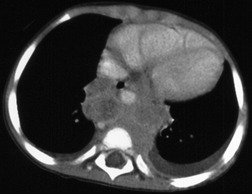 |
| Fig. 14.88 (Courtesy of Donald Frush, MD, Durham, NC.) |
CT can show spinal and intraspinal involvement (Fig. 14.81),510. and 511. particularly if intrathecal contrast has been administered (rarely performed). However, MRI is considered the standard for imaging neurogenic tumors because it better demonstrates spinal and intraspinal involvement (Fig. 14.81).404. and 512. The signal pattern at MRI is variable (Fig. 14.89, Fig. 14.90 and Fig. 14.91). Neurogenic tumors may show uniform signal intensity similar to muscle on T1-weighted sequences and signal intensity considerably higher than muscle on T2-weighted sequences. Neurofibromas, on occasion, show the so-called ‘target pattern’ with different signal in the central portion of the lesion compared with the periphery (see Fig. 14.79).507.513.514. and 515. On T1-weighted images, the central portion is of higher signal, whereas on T2-weighted spin-echo images, the periphery is of higher intensity than the center, corresponding to the histopathologic finding of central nerve tissue and peripheral myxoid degeneration. 516 Schwannomas and ganglioneuromas may show heterogeneous high signal intensity throughout the lesion on T2-weighted images, and low to intermediate signal intensity on T1-weighted images. 32 In these cases, the signal high intensity on T2-weighted images is probably due to cystic degeneration.507. and 516. Ganglioneuromas may have a whorled appearance on MRI, corresponding to whorls of collagenous fibrous tissue and neural tissue.
Because most neurogenic tumors in adults are benign, the role of imaging is to facilitate differential diagnosis and to evaluate local extent prior to resection. MRI is probably the technique of choice for imaging neurogenic tumors because it best shows intraspinal extension. For malignant tumors, notably neuroblastoma, chest radiography and MRI appear to be the best imaging techniques for staging (Fig. 14.91).502. and 517. Radionuclide imaging with agents such as meta-iodobenzylguanidine (MIBG) or FDG-PET scanning can also be used to assess the extent of tumor and for staging.518. and 519. FDG-PET imaging is likely of little benefit for differentiation of benign from malignant nerve sheath tumors, as benign schwannomas may show increased metabolic activity (Fig. 14.82).520. and 521.
Mediastinal paragangliomas
Paragangliomas (Box 14.16) are rare tumors that arise from chromaffin cells and may be either benign or malignant.522.523. and 524. Most such tumors arise in the adrenal glands and are known as pheochromocytomas. Those that arise elsewhere are known collectively as paragangliomas. 523 Mediastinal paragangliomas are rare, constituting less than 2% of thoracic neurogenic tumors in one large series. 487 In a review of 51 mediastinal paragangliomas, two-thirds arose in the region of the aortic arch (aortic body tumors) and one-third arose in the paravertebral region (Figs 14.92 and 14.93). 525 Aortic body tumors may occur in one of four locations: lateral to the brachiocephalic artery; anterolateral to the aortic arch; at the angle of the ductus arteriosus; or above and to the right of the right pulmonary artery.524. and 525. Tumors may also arise in the wall of the left atrium or the interatrial septum (Fig. 14.94).526.527. and 528. Multifocal lesions are also reported. 529 Most mediastinal paragangliomas are nonfunctional and are detected either incidentally or because of signs and symptoms related to compression or invasion of mediastinal structures. 524
Mediastinal paragangliomas manifest as round soft tissue masses on CT that, because they are highly vascular, can enhance intensely after administration of intravenous contrast.30. and 524. Smaller lesions tend to be of homogeneous attenuation, while larger lesions may be more heterogeneous, due to necrosis (Fig. 14.92). 524 Arteriography demonstrates enlarged feeding vessels, pathologic vessels within the tumor, and an intense tumor blush. 530 Radioiodine MIBG (Fig. 14.93) and somatostatin receptor scintigraphy all show increased activity in paragangliomas.526.531.532.533. and 534.
The MR findings of thoracic paragangliomas are the subjects of case reports only.535.536. and 537. Based on these reports, it seems that the MR appearance of thoracic lesions is similar to that of lesions more commonly encountered in the head and neck: the masses are isointense to muscle on T1-weighted images and are of substantially higher signal than muscle on T2-weighted images (Fig. 14.95). 538 Numerous serpiginous vascular channels may also be seen coursing through the larger lesions. MRI is particularly advantageous for showing intracardiac paragangliomas (Fig. 14.94). Lesions can be metabolically active on FDG-PET scans (Fig. 14.92).
Parathyroid lesions of the mediastinum
Primary hyperparathyroidism is usually caused by a parathyroid adenoma (Box 14.17) in the neck. Surgeons frequently do not obtain preoperative imaging studies to localize the parathyroid glands because neck exploration is curative in over 90% of affected patients. 539 However, about 10% of adenomas arise in ectopic parathyroid glands in the mediastinum, usually in or around the thymus gland.540. and 541. In one large series, the two most common ectopic locations were intrathymic and paraesophageal. 542 Affected patients may have four parathyroid glands in the normal position in addition to the ectopic mediastinal adenoma. Although the ectopic adenoma is usually solitary, at least one patient with multiple mediastinal adenomas has been reported. 543
Box 14.17
Ectopic parathyroid glands
• 10% parathyroid adenoma
• Arise near thymus gland, paraesophageal
• 99mTc-sestamibi findings
– Technique of choice for detection
– Sensitivity 90%, especially with single photon emission computed tomography (SPECT)
• CT findings
– Homogeneous soft tissue attenuation
– <2.0 cm diameter
– May enhance
– Used to localize lesions detected by 99mTc-Sestamibi
• MRI
– Intermediate T1 signal
– Hyperintense T2 signal (90%)
– Isointense T2 signal (10%) due to fibrosis
Parathyroid cyst
• Very rare
• Usually discovered incidentally
• One-third has hyperparathyroidism
• Superior mediastinal cystic lesion, thin enhancing rim
As ectopic adenomas can be missed at surgical exploration, preoperative localization with imaging studies can reduce operative time, postoperative morbidity, and requirement for repeat surgery. 404 Imaging techniques for localizing ectopic mediastinal parathyroid glands include radionuclide imaging (99mTc-MIBI, 99mTc-tetrofosmin),544. and 545. CT, and MRI.546.547. and 548. Mediastinal parathyroid glands are probably best demonstrated using 99mTc-sestamibi radionuclide imaging.544.545.546.547.549.550. and 551. Sensitivities of over 90% are reported, especially with use of SPECT. 552 CT or MRI are usually reserved for anatomic localization of an abnormality detected on the 99mTc-sestamibi scan. 404 However, combined CT/SPECT imaging can also be used to improve localization of mediastinal lesions. 553
On noncontrast CT, mediastinal parathyroid adenomas manifest as small (usually less than 2 cm diameter) homogeneous masses of soft tissue attenuation (Fig. 14.96). Diagnosis on noncontrast CT can be difficult as they are easily confused with normal mediastinal lymph nodes, thymic remnants, or blood vessels. Diagnosis on contrast-enhanced CT is usually more straightforward, although the lesions may sometimes enhance intensely. It is important to realize that a mediastinal parathyroid adenoma may be found as low as the aortopulmonary window and, therefore, the search for such lesions must extend at least to the level of the tracheal carina. 554
On MRI, parathyroid adenomas are usually of intermediate signal intensity on T1-weighted MR images and of markedly increased signal intensity on T2-weighted images (Fig. 14.97). 555 However, up to 13% of abnormal glands do not have high signal intensity on T2-weighted images, possibly due to fibrosis or hemorrhage.404. and 555. MR has comparable sensitivity (78%) and specificity (90%) with other imaging techniques for detecting parathyroid pathology, but its most appropriate use is as an adjunct to 99mTc-MIBI radionuclide imaging. 555 MRI provides accurate anatomic localization of the adenoma and can predict the need for mediastinotomy or lateral cervical incision. MRI is also useful in ‘second-look operations’ and in high-risk patients. In such patients, the success rate for surgery is reduced and the combined use of 99mTc-MIBI and MRI is 89% sensitive and 95% specific for preoperative localization. 549
Mediastinal parathyroid cysts are quite rare. 556 Most are discovered incidentally, although they can compress the trachea or recurrent laryngeal nerve, causing symptoms.557. and 558. About a third of patients present with hyperparathyroidism. 556 Adenomas are occasionally cystic as well. 559 The radiographic, CT, and MRI findings are those of a superior mediastinal cystic mass with a thin, enhancing rim (Fig. 14.98). Adjacent structures may be displaced and deformed.
Pneumomediastinum
The presence of gas in the mediastinum (pneumomediastinum) indicates perforation of a portion of either the respiratory or alimentary tracts. Perforation need not be within the mediastinum; indeed, it often lies beyond the confines of the mediastinum itself. Gas-forming mediastinal infection is a very rare cause of air within the mediastinum. The causes of pneumomediastinum are shown in Box 14.18. 560
Box 14.18
Alveolar rupture
• Patients on mechanical ventilation
• Compressive trauma to the thorax
• Laceration of lung by rib fracture
Miscellaneous causes
• Trauma to trachea or central bronchi (see Chapter 17)
• Perforation of esophagus
• Postoperative
– Placement of an intercostal chest tube
– Mediastinoscopy
– Mediastinal surgery
Patients with pneumomediastinum due to spontaneous alveolar rupture are usually young and have a history of asthma or severe coughing or vomiting.561. and 562. Conditions reported in association with spontaneous pneumomediastinum96.560.561. and 563. include asthma, croup, 564 strenuous exercise, marijuana or crack cocaine smoking,565. and 566. nitrous oxide inhalation, 567 pneumonia, diabetic ketoacidosis, 568 pulmonary fibrosis, 569 and childbirth. When spontaneous alveolar rupture occurs, air dissects through the pulmonary interstitium into the mediastinum. 570 Air may also rupture into the pleural space producing pneumothorax. The patient may complain of chest pain aggravated by deep breathing and dyspnea. Fever and leukocytosis without apparent infection are also frequently noted561 and may cause diagnostic confusion with acute mediastinitis. Spontaneous pneumomediastinum due to alveolar rupture, though it may cause symptoms, does not usually adversely affect patient outcome, and is therefore not treated560. and 571. unless accompanied by pneumothorax. However, on rare occasions, air may collect in the mediastinum under pressure (tension pneumomediastinum) and cause impairment of venous return.572. and 573. In these rare cases, percutaneous catheter drainage can be lifesaving.572. and 573.
Alveolar rupture is also common in patients who are on mechanical ventilation, particularly those with small airway obstruction or noncompliant lungs574 due to hyaline membrane disease, meconium aspiration, neonatal pneumonia, or acute respiratory distress syndrome. In these patients, pneumomediastinum itself rarely affects patient outcome and is not treated, but it may be a harbinger of more serious and immediate problems such as pneumothorax. In such cases, reduction of ventilatory pressures to the minimum needed is advisable.
Air in the chest wall following rib fracture or placement of a chest tube may dissect into the mediastinum, usually by way of the neck. This is particularly common in patients on mechanical ventilation. Usually the chest wall emphysema is severe, and the cause of the pneumomediastinum is not in doubt. Bubbles of air within the mediastinum are often seen on the first day following mediastinoscopy. In one series of 10 patients examined serially following uncomplicated mediastinoscopy these bubbles had all cleared by the third day and fluid levels were never seen. 575 Air is also common in the mediastinum after cardiac surgery and may persist for days.
Radiographic findings of pneumomediastinum
Pneumomediastinum manifests on chest radiographs as streaks, bubbles, or larger collections of gas outlining mediastinal blood vessels, major airways, esophagus, or diaphragm (Fig. 14.99, Fig. 14.100, Fig. 14.101 and Fig. 14.102).96.430. and 563. Dissection of air along tissue planes may be more obvious on the lateral projection than on the frontal view. The air may dissect under the parietal layer of the mediastinal pleura so that a thick linear opacity representing the combined parietal and visceral pleura can be seen along the heart and great vessels. Air may also dissect extrapleurally over the apices, simulating the appearance of pneumothorax. The air is usually greatest in amount anteriorly. When limited in volume, the only sign of pneumomediastinum on chest radiography may be a lucent line or bands seen in the retrosternal area.
An important radiographic finding of pneumomediastinum is air dissecting under, and medial to, the thymus. The outlining of the thymus by air is quite specific for pneumomediastinum and may be the most striking sign of the condition (Fig. 14.99). Air may also track extrapleurally along the upper surface of the diaphragm. Air between the heart and diaphragm gives rise to the ‘continuous diaphragm sign’ (Fig. 14.101) 583– so-called because air beneath the heart may form a visible lucent line that permits the entire upper surface of the diaphragm to be seen, even within the mediastinum.
It may, on occasion, be difficult to distinguish pneumomediastinum from pneumothorax or pneumopericardium on chest radiographs (Fig. 14.102). The distinction depends on the anatomic extent of the air. 96 Pneumothorax is rarely confined solely to the mediastinal border. It can usually be traced out over the lung apex to the lateral portion of the thoracic cavity. A lateral decubitus view can help confirm that the visualized air is within the pleural space by more clearly delineating a visceral pleural line, but it is rarely required. Pneumopericardium may extend from the diaphragm to just below the aortic arch, but will not extend around the aortic arch or into the superior mediastinum. Pneumopericardium can mimic the continuous diaphragm sign and can lift the thymus away from the great vessels, but the bilateral nature of the air and anatomic conformity to the pericardium is usually evident. A thin line of apparent radiolucency is frequently seen along the heart borders and aorta in healthy individuals due to the ‘Mach band’ phenomenon. The Mach band may have an identical degree of radiolucency to a small pneumomediastinum. The distinction from pneumomediastinum584 depends on analyzing both the anatomic extent and the border of the radiolucent line. A Mach band will be adjacent to a normally visualized contour, and its lateral boundary will either be unrecognizable or will be formed by a pulmonary blood vessel. The outer margin of a pneumomediastinum, on the other hand, will be the displaced mediastinal pleura.
The diagnosis of mediastinal emphysema is made readily at CT (Fig. 14.100), as the anatomic location of the air is self-evident on cross-sectional images. 563 CT is both more sensitive and specific than chest radiographs for the diagnosis of pneumomediastinum and can be used to confirm the diagnosis in patients with clinical suspicion of pneumomediastinum when the chest radiograph is normal or equivocal. This is usually only necessary in patients with suspected rupture of the trachea or central bronchi or in cases of suspected esophageal perforation.
Sarcomas of the mediastinum
Primary mediastinal sarcomas are uncommon585. and 586. and include fibrosarcoma, osteosarcoma, 587 chondrosarcoma, 588 rhabdomyosarcoma, 589 synovial sarcoma, 590 follicular dendritic cell sarcoma, 591 and liposarcoma592 (see p 896). Chest wall tumors, such as chondrosarcoma or giant cell tumor, may project into the mediastinum and simulate the appearance of a mediastinal mass on chest radiographs or CT (Fig. 14.103). 593 Chordomas are usually tumors of the spinal canal but are occasionally found in the posterior mediastinum.594. and 595.
Patients with mediastinal sarcomas may be asymptomatic at presentation, or may present with complaints due to compression or invasion of mediastinal structures such as the superior vena cava or trachea. On chest radiographs, they manifest as smooth or lobulated masses that may have either well-defined or poorly defined margins. On CT, they are usually heterogeneous soft tissue attenuation masses (Fig. 14.104) and may show extensive local invasion. As such, these lesions are generally indistinguishable from other soft tissue attenuation mediastinal masses. The diagnosis of osteosarcoma or liposarcoma may be suggested by the presence of extensive ossification or fat, respectively, within the lesions.586. and 587.
Superior vena cava syndrome
The commonest cause of superior vena cava obstruction is compression and direct invasion by bronchogenic carcinoma (see Box 14.19).596.597.598. and 599. Other causes include mediastinal tumors, notably metastatic breast and testicular neoplasms and lymphoma, fibrosing mediastinitis, and thrombosis due to venous catheters. 596 In one large review, 78% of cases were due to malignant neoplasms (two-thirds of which were lung carcinomas) and 22% were due to benign causes. 596
Box 14.19
Malignant (78%)
• Lung cancer
• Lymphoma
• Germ cell malignancy
• Metastatic disease
Benign (22%)
• Fibrosing mediastinitis
• Thrombosis (e.g. caused by central venous catheters)
• External compression by
• Aneurysms
• Mediastinal cysts
• Thoracic surgery
Clinically, the features of superior vena cava obstruction are edema and visible distension of the veins of the face, neck, arms, and anterior chest wall. Dyspnea, choking, dysphagia, and a feeling of congestion are common symptoms. Cerebral edema may rarely develop. The severity of the symptoms and signs depends on the degree of venous collateral formation. 600 There may be no symptoms at all, even with complete superior vena cava obstruction, if the obstruction develops slowly and numerous collaterals form.
Many imaging techniques can be used to diagnose superior vena cava obstruction.597. and 601. On occasion, the presence of obstruction can be inferred from the radiographic appearance of dilatation of collateral venous structures such as the azygos or left superior intercostal vein (aortic nipple) which drains the hemiazygos system (Fig. 14.105). 602 In cases of superior vena cava thrombosis around an indwelling catheter, the superior mediastinum may become visibly widened on chest radiography. 603 This observation may allow a diagnosis of thrombosis even when it is not suspected clinically. 603 Up to 60% of patients have pleural effusions that are usually small and show no left or right preference. 604




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree