LUNG CANCER
The American Cancer Society estimated that in the USA, in 2007, there would be 215 000 new cases of lung cancer and 162 000 related deaths. In contrast, colorectal, breast, and prostate cancers combined would be responsible for only 124 000 deaths. 1
Pathology
The most widely accepted histologic classification of lung cancer is that of the World Health Organization (WHO). 2 The common types are (see Box 13.1):
• Squamous cell carcinoma (SCC), which accounts for 30–35% of cases. Its relative incidence appears to be falling, probably because the prevalence of smoking is declining. Histologic diagnosis of squamous cell carcinoma is based on the presence of keratin production by tumor cells or intercellular desmosomes, so-called ‘intercellular bridges’. Tumors that are either predominantly spindled or have a characteristic histologic pattern of peripheral palisading may also be classified as SCC.
• Adenocarcinoma, which accounts for up to 35% of cases. Its relative incidence is rising. More recent reports have noted a shift in the relative frequency of lung carcinoma tumor types such that adenocarcinoma now is more common than squamous cell carcinoma, notably in women. 2 A small proportion of adenocarcinomas show neuroendocrine features, which confer greater aggressiveness to the tumor. 3
• Large cell carcinoma (LCC), which accounts for 10–15% of cases. LCC is a malignant epithelial neoplasm lacking glandular or squamous differentiation by light microscopy and lacking cytologic features of small cell carcinoma. As the definition implies, LCC is a diagnosis of exclusion intended to include all poorly differentiated nonsmall cell lung cancers (NSCLCs) that are not further classifiable by routine light microscopy. 2 They may show neuroendocrine differentiation, in which case the prognosis is comparable to small cell carcinoma.4. and 5.
• Small cell carcinoma, which accounts for 20–30% of cases. Small cell lung carcinomas (SCLCs) grow rapidly and metastasize early. They contain neurosecretory granules and are part of a spectrum of neuroendocrine tumors. SCLC shows a strong correlation with cigarette smoking and is extremely rare in persons who have never smoked. Molecular studies have identified a number of abnormalities in SCLCs, including in particular deletions in chromosome 3p. 2
Box 13.1
Squamous cell carcinoma
• Papillary
• Clear cell
• Small cell
• Basaloid
Small cell carcinoma
• Combined small cell carcinoma
Adenocarcinoma
• Acinar
• Papillary
• Bronchioloalveolar carcinoma
– Nonmucinous (Clara cell/type II pneumocyte)
– Mucinous (goblet cell type)
– Mixed mucinous and nonmucinous (Clara cell/type II pneumocyte/goblet cell type) or indeterminate
• Solid adenocarcinoma with mucin formation
• Mixed
• Variants
– Well-differentiated fetal adenocarcinoma
– Mucinous (‘colloid’)
– Mucinous cystadenocarcinoma
– Signet ring
– Clear cell
Large cell carcinoma
• Large cell neuroendocrine carcinoma
– Combined large cell neuroendocrine carcinoma
• Basaloid carcinoma
• Lymphoepithelioma-like carcinoma
• Clear cell carcinoma
• Large cell carcinoma with rhabdoid phenotype
Adenosquamous carcinoma
Carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements
• Carcinomas with spindle and/or giant cells
– Pleomorphic carcinoma
– Spindle cell carcinoma
– Giant cell carcinoma
• Carcinosarcoma
• Blastoma
Carcinoid tumor
• Typical carcinoid
• Atypical carcinoid
Carcinomas of salivary gland type
• Mucoepidermoid carcinoma
• Adenoid cystic carcinoma
• Others
Unclassified carcinoma
The histologic distinction among the nonsmall cell carcinomas is not always clear-cut: pathologists may differ in their interpretations, and different portions of the same tumor may warrant different classifications. Although individual cases may require special diagnostic studies for accurate diagnosis, classification of conventional bronchogenic carcinomas is usually accomplished on the basis of routinely stained cytologic or histologic samples. The WHO classification of lung tumors, revised in 2004, is the foundation for the nomenclature. 2 The new classification aims to account for the recognition of lung carcinoma heterogeneity, the introduction of diagnostic immunohistochemical staining, and the recognition of newly described entities such as fetal adenocarcinoma, cystic mucinous tumors, and large cell neuroendocrine carcinoma.
Cigarette smoking is the most important single risk factor for lung cancer.6. and 7. Other risk factors include asbestos exposure, 8 radiation therapy, environmental factors, dietary factors, human immunodeficiency virus (HIV) infection, genetic factors, and pulmonary fibrosis.9. and 10. A discussion of the epidemiologic and causative factors of lung cancer is beyond the scope of this book, but several reviews suitable for radiologists have been published.6.11. and 12. In one series, lung cancer was found in 32 out of 244 patients with idiopathic pulmonary fibrosis. The carcinomas were often ill-defined lesions mimicking airspace consolidation, corresponding to the location of the most advanced fibrosis. 13 Heart or lung transplantation may also predispose to lung cancer, the resulting tumors showing typical radiographic features.14. and 15. It is no longer accepted that lung cancers in general arise from old tuberculous scars. In most so-called focal scar carcinomas the fibrous tissue is probably a desmoplastic response to the cancer; in other words the scar follows, rather than precedes, the development of carcinoma.16. and 17. Gender differences in frequency of tumor subtypes and susceptibility are increasingly recognized.10. and 18.
Clinical features
The majority of patients with lung cancer have advanced disease at clinical presentation. This may reflect the aggressive biology of the disease and the frequent absence of symptoms until the disease is locally advanced or metastatic.
The symptoms vary with extent of disease. 19 Cough, dyspnea, occasionally associated with wheeze, mild hemoptysis, recurrent pneumonia, and paraneoplastic syndromes (see Table 13.1) are the cardinal symptoms of the disease at a stage when the carcinoma is still confined to the lung and major bronchi. Hoarseness, chest wall pain, brachial plexus neuropathy, Horner syndrome, phrenic nerve paresis, superior vena caval obstruction, dysphagia, and the symptoms due to pleural effusion or pericardial tamponade indicate invasion of the mediastinum, pleura, pericardium, or chest wall. 20 Peripheral and slow-growing tumors are clinically silent for a longer period and more likely to be discovered incidentally on chest radiographs or computed tomography (CT) examinations.
| *Modified from Beckles et al.19and Filderman et al.21 |
| Endocrine and metabolic Cushing syndrome Inappropriate secretion of antidiuretic hormone Carcinoid syndrome Hypercalcemia and ectopic parathyroid hormone secretion Hypercalcitonemia Ectopic gonadotropin, gynecomastia, male gonadal dysfunction Hypoglycemia Acromegaly Hyperthyroidism Cachexia of malignancy Lactic acidosis Hypouricemia |
| Neurologic and neuromuscular Eaton–Lambert syndrome Polymyositis Mononeuritis multiplex Subacute cerebellar degeneration Encephalomyelopathy Retinopathy |
| Skeletal Clubbing Pulmonary hypertrophic osteoarthropathy Osteomalacia |
| Renal Glomerulonephritis Nephrotic syndrome |
| Dermatologic Acanthosis nigricans Scleroderma Hypertrichosis lanuginosa Erythema gyratum repens Erythema multiforme Tylosis Exfoliative dermatitis Sweet syndrome Other dermatoses Urticaria Pruritis |
| Vascular Migratory thrombophlebitis Disseminated intravascular coagulation Arterial thrombosis Nonbacterial verrucous endocarditis |
| Hematologic Anemia Red cell aplasia Thrombocytopenic/fibrinolytic purpura Nonspecific leukocytosis Thrombocytosis Polycythemia Eosinophilia Leukoerythroblastic reaction |
| Systemic Anorexia Cachexia Fever |
The clinical symptoms and signs vary with cell type. 21 Squamous cell carcinoma is a relatively slow-growing, late-metastasizing tumor that most often arises centrally within the bronchial tree and usually manifests as obstructive atelectasis or pneumonia, hemoptysis, or the signs and symptoms of invasion of adjacent structures, such as recurrent laryngeal nerve paralysis. When squamous cell cancers arise peripherally in the lung, they may grow to a substantial size before symptoms develop.
Adenocarcinoma most often arises as a peripheral pulmonary nodule and is frequently first discovered on a chest radiograph in a patient with no chest symptoms. Nevertheless, hilar and mediastinal node involvement and distant metastases, particularly to the brain and adrenal glands, are frequently present at or soon after presentation. Dyspnea resulting from pleural effusion is a particular feature of adenocarcinoma. Bronchioloalveolar carcinoma when it presents as a solitary pulmonary nodule or mass, unlike other bronchial adenocarcinomas, is often an indolent tumor that metastasizes late.
Large cell carcinoma is similar to epidermoid carcinoma in that the tumor may grow to a large size, but dissimilar in that it metastasizes early, particularly to the mediastinum and brain. Small cell carcinoma also metastasizes early and widely; metastases are usually present at initial diagnosis. Hormone production, notably adrenocorticotrophic hormone, antidiuretic hormone, and melanocyte-stimulating hormone, is a feature of small cell tumors. 22
Imaging
The imaging appearances of lung cancer are considered in the following framework: (1) peripheral tumors (tumors arising beyond the hilum/segmental bronchi); and (2) central tumors (tumors arising at or close to the hilum/segmental bronchi).
Peripheral tumors (see Box 13.2)
Approximately 40% of lung cancers arise beyond the segmental bronchi, 23 and in 30% a peripheral mass is the sole radiographic finding.24.25. and 26. The fact that peripheral tumors tend to grow predominantly in the direction of the hilum may explain the variable proportions of peripheral versus central tumors reported in large surveys and the unexpectedly high number of ‘peripheral’ masses visible at bronchoscopy. The mass can be virtually any size, but it is rare for a lung cancer to be seen on chest radiographs unless it is more than l cm in diameter.27. and 28. CT, because of its better contrast resolution, detects much smaller lesions.29. and 30.
Box 13.2
• Approximately 40% of lung cancers arise beyond segmental bronchi
• Rarely visible on chest radiographs when below 1 cm in diameter
• Usual shapes are spherical, oval, or lobulated
• Edge is usually lobular or irregular, but may, rarely, be ill-defined enough to resemble pneumonia
• A ‘corona radiata’ is nearly specific for lung cancer
• May present as mucocele (mucoid impaction, bronchocele)
• Cavitation is seen in 16% of cases on chest radiographs and more frequently on CT
• Air bronchograms and cystlike lucencies are rarely visible on chest radiographs, though they are seen in 25% of cases on CT
• Visible calcification is virtually never identified on chest radiography but is seen in a small proportion of cases on CT
• Volume doubling times are very rarely less than 1 month or more than 18 months
Shape
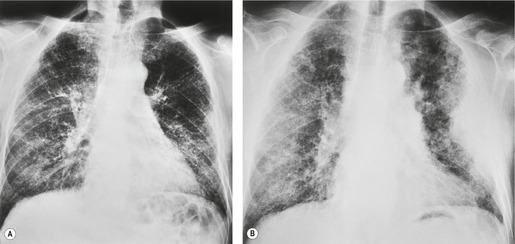
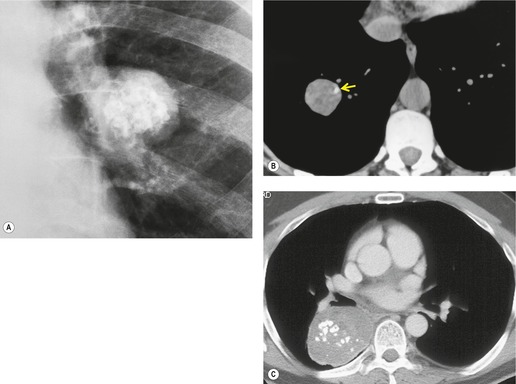
In general, peripheral lung cancers assume an approximately spherical or oval configuration, unless they are limited by an unyielding surface (Fig. 13.1). The major exceptions are tumors at the lung apex, such as Pancoast and superior sulcus tumors, which may resemble apical pleural thickening, certain bronchioloalveolar carcinomas, and carcinomas arising in areas of interstitial fibrosis. Bronchogenic carcinoma is therefore one of the major diagnostic considerations in adults with a solitary pulmonary nodule.30. and 31.
Lobulation, a sign that indicates uneven growth rates for differing portions of the tumor, is common. 32 An equally frequent sign is a notch (umbilication), a sign that is the counterpart of lobulation because it indicates relatively slow growth of a particular portion of the tumor. Occasionally a dumbbell shape is encountered or two nodules are seen next to each other. 31
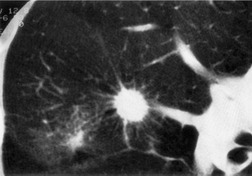
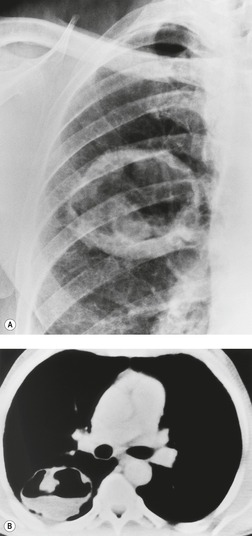
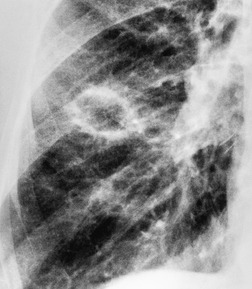
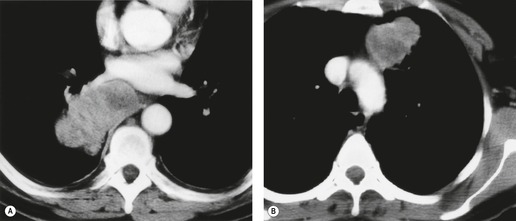
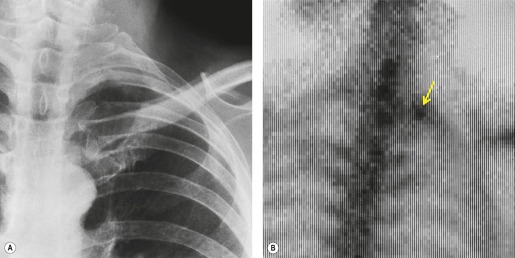
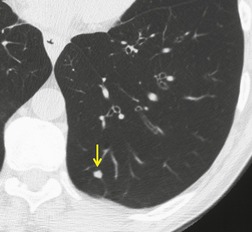
Sometimes the edge of the tumor is irregular, with one or more strands radiating into the surrounding lung, an appearance often described as spiculated (Fig. 13.2). The term ‘corona radiata’33 indicates multiple strands extending into the surrounding lung because of either tumor extension or a fibrotic response to the tumor. Such stranding is best seen at CT (Figs 13.2 and 13.3).31. and 34. Coarse spiculation has been shown, in adenocarcinoma at least, to confer a worse prognosis than a smooth or lobulated outline. 35 A well-developed corona radiata is a useful sign in the differential diagnosis of a solitary pulmonary nodule because it makes the diagnosis of lung cancer highly likely. It is not, however, entirely specific: similar very irregular margins are encountered in a variety of lesions, including benign processes, notably chronic pneumonia and granuloma. 31 A single linear or bandlike opacity may connect the lesion to the pleura (Fig. 13.3). This so-called ‘pleural tail sign’ is seen with both benign and malignant pulmonary nodules.30. and 31.
Careful observation of the pattern of vessels in the neighboring lung parenchyma may show convergence of peripheral blood vessels leading to and entering the cancerous mass (Fig. 13.3), a sign that is best appreciated on CT. 34 In one small series using multiplanar reconstructions, all 15 lung cancers showed direct involvement of a pulmonary vein. 36
Regardless of the irregularity of the border, the nodules or masses described thus far can be regarded as having a well-defined edge. Some cancers show a poorly defined edge, similar to that seen in pneumonia (Figs 13.4 and 13.5). In such cases the spherical shape and relatively slow growth usually allows distinction from infectious processes, which in general change size within a few weeks. 32
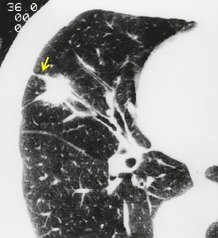
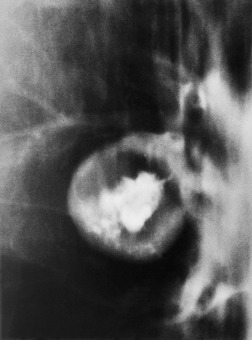
Occasionally, lung cancers arising in segmental or subsegmental bronchi are seen radiographically as mucoid impaction, resembling mucoceles or bronchoceles (Fig. 13.6), 37 in which case the resulting opacity, to a greater or lesser extent, represents dilated bronchi filled with tumor or inspissated secretions. For a mucoid impaction to be visible on chest radiographs, the adjacent lung must be aerated by collateral air drift. Mucoid impactions result in V-shaped, Y-shaped, or branching tree densities with their stems pointing towards the hilum. Another rare pattern is an infarct extending from the primary tumor, giving two contiguous but distinct components to the opacity, the distal one being a pleural-based infarct. 38 Finally, hematogenous tumor spread may mimic various embolic patterns. 39
Calcification and cavitation
Most lung cancers are of soft tissue density on CT, but some show partial calcification and some show cavitation. 40
Calcification
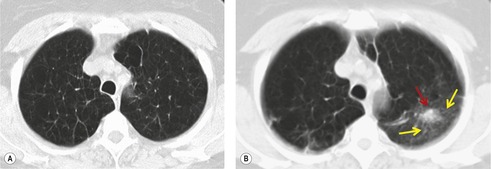
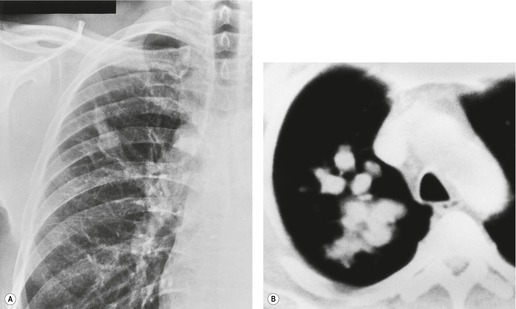
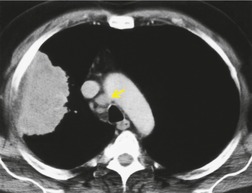
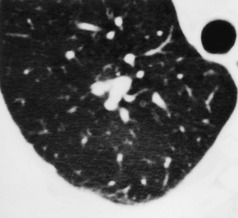
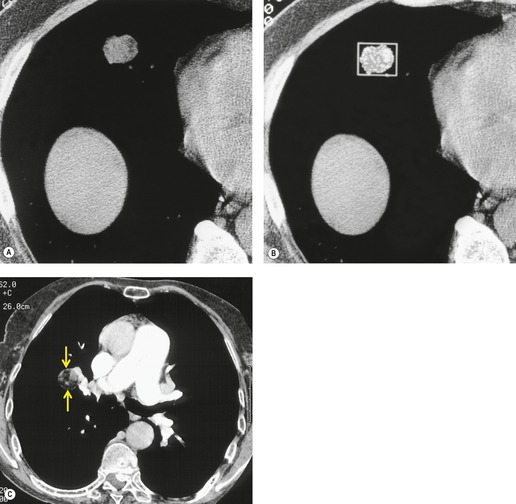
Pathologists have long recognized that calcification is seen on histologic examination of lung cancers. Such calcification may be dystrophic in areas of tumor necrosis or may be an intrinsic part of the tumor. Tumor calcification is rarely seen on radiographs, except at specimen radiography. 41 CT shows calcification within 6–10% of bronchogenic carcinomas. 42 Mostly, the calcifications represent preexisting granulomatous calcifications engulfed by the tumor. However, amorphous or cloudlike calcification, in keeping with dystrophic tumor calcification, is seen in a significant proportion.31. and 42. Both small cell and nonsmall cell carcinomas may calcify, and there does not appear to be a predilection for cancers of any particular cell type. 43 Most tumors which show calcification are large with a diameter of 5 cm or more, 44 but amorphous or cloudlike calcification can also be seen in small peripheral tumors (Fig. 13.7). Granulomatous calcifications engulfed by the tumor are likely to be eccentric in location (Fig. 13.8), but granulomatous calcifications are occasionally seen.
Cavitation
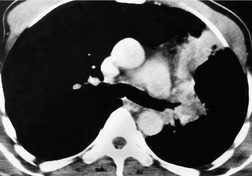
Cavitation may be seen in tumors of any size and is best demonstrated by CT, which may show air, liquefaction or a mixture of the two. The cavity is frequently eccentric, the walls are often irregular, and tumor nodules may be visible (Fig. 13.9). The wall is usually thick, but can be thin or of variable thickness (Figs 13.10 and 13.11).45. and 46. Cavitating lung cancer may even have smooth inner and outer margins. It has been suggested that such very thin-walled cavities represent tumor cells lining bullae rather than true cavitation. 47 Fluid levels are common, and necrotic tumor fragments may be seen within the cavity. Very rarely, the cavity shows as an air crescent, similar to a mycetoma, caused by air around an intracavitary tumor mass or formed debris. 48
 |
| Fig. 13.11 (Courtesy of John Anthony Parker, Boston, MA, USA.) |
Approximately 16% of peripheral carcinomas show cavitation on chest radiographs. 26 The incidence is clearly much higher on CT. 31 Squamous cell carcinoma is much more likely to cavitate than cancers of other cell types. In one series of 100 cavitating cancers, 82 were squamous cell lesions. 47 Adenocarcinomas and large cell carcinomas cavitate occasionally, whereas small cell carcinomas, for practical purposes, do not cavitate.47. and 49. An appearance closely resembling a pulmonary cyst has been described in mucinous cystadenocarcinoma. 50
Ground-glass density, air bronchograms, and cystlike lucencies
Ground-glass density
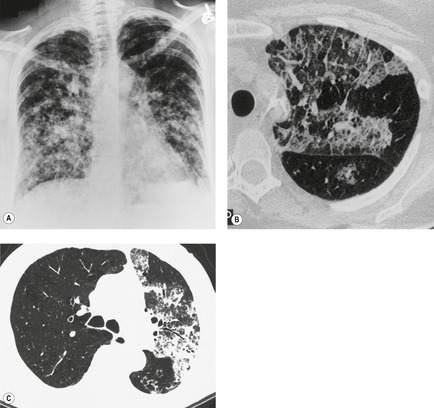
Screening studies have shown a particularly high incidence of malignant nodules showing ground-glass attenuation. 51 The most common pathology in these lesions is adenocarcinoma with a bronchioloalveolar cell component or bronchioloalveolar carcinoma. 51 Because positron emission tomography (PET) is often negative in these lesions given their low metabolic activity, biopsy or resection is often recommended, notably in high-risk patients. 31 The proportion of the nodule showing ground-glass opacity correlates with both cell type and histologic features. In an analysis of 124 surgically resected, peripheral adenocarcinomas less than 2 cm in diameter, the proportion of ground-glass opacity in individual lesions was significantly greater in bronchioloalveolar carcinoma than in other types of adenocarcinoma. 52 Minor degrees of ground-glass opacity are seen in other cell types due to adjacent inflammation, edema, or hemorrhage. 53 Aoki et al. 35 compared the extent of vessel invasion and regional node metastases with the margin characteristics and extent of ground-glass opacity on CT of 127 peripheral adenocarcinomas less than 3 cm in diameter. They found that both vessel invasion and regional lymph node spread were significantly lower when the ground-glass component of primary tumor was greater than 50%. They also showed that tumors with ground-glass components of more than 50% had a statistically significantly better prognosis than those tumors whose solid component comprised more than 50%. The same authors54 also approached the problem of prognosis in a slightly different way by showing that the greater the proportion of ground-glass opacities in adenocarcinomas, the slower the growth rate. In a different study, 55 236 surgically resected small peripheral adenocarcinomas measuring 2 cm or less in greatest diameter were reviewed using a histologic classification based on tumor growth patterns. The authors described six morphological patterns: type A (localized bronchioloalveolar carcinoma, LBAC), which revealed replacement growth of alveolar-lining epithelial cells with a relatively thin stroma; type B (LBAC with foci of structural collapse of alveoli), which showed fibrotic foci due to alveolar collapse; type C (LBAC with foci of active fibroblastic proliferation), which was the largest group and showed foci of active fibroblastic proliferation; type D (poorly differentiated adenocarcinoma); type E (tubular adenocarcinoma); and type F (papillary adenocarcinoma with a compressive growth pattern), which showed compressive and expanding growth. Types A and B showed no lymph node metastasis and had the most favorable prognosis of the six types. 55 The detection of these nodules on CT examinations as well as potential follow-up strategies and management recommendations are among the intensely discussed topics in the current literature.56.57.58.59.60.61.62.63. and 64.
Air bronchograms and cystlike lucencies
Lung cancers, particularly adenocarcinomas and bronchioloalveolar carcinomas, may contain air bronchograms or cystlike lucencies. Air bronchograms are usually thought of as a feature of infective consolidation or other alveolar filling processes. Kuriyama et al., 65 however, found that with CT some air-filled bronchi or bronchioles could be identified within the majority of peripherally situated small adenocarcinomas. Cystlike areas of low attenuation are seen fairly frequently: they were identified in 25% of one series of 93 cases of a solitary pulmonary nodule caused by lung cancer. 66 These lucencies, which are seen in lung cancers of all cell types, but are most frequently encountered in adenocarcinoma and bronchioloalveolar carcinoma,67. and 68. are due either to patent small bronchi or to small cystic spaces within the tumor. An abruptly obstructed bronchus entering a tumor is a common feature of squamous cell carcinoma. 67
Contrast enhancement
Contrast enhancement reflects tumoral vascularity. Tateishi et al. 69 correlated dynamic CT contrast-enhancement with pathologic findings and the vascular endothelial growth factor (VEGF) expressed by various resected adenocarcinomas and showed that dynamic CT reflects the microvessel density of adenocarcinomas of the lungs.
Peripheral lung cancers enhance following the intravenous injection of contrast agents at both CT and magnetic resonance imaging (MRI). This phenomenon has been exploited in an attempt to distinguish lung cancer from other causes of a solitary pulmonary nodule.70.71. and 72. To facilitate comparisons of measurements between and within patients, a standardized perfusion value has been proposed, and this correlates well with metabolic activity on PET. 73
Rate of growth
The absence of growth over a 2-year period is a relatively reliable indicator for a benign nature of a nodule, although this commonly used criterion is not absolutely reliable.29. and 74. To establish the absence of growth, radiographs with comparable techniques are necessary. Radiographic exclusion of growth can also be supported by digital analysis techniques.75. and 76. A calculation of lesion doubling time, which for a spherical lesion can be approximated to a 25% increase in diameter, has been used to distinguish benign from malignant lesions. This was based on the observation that benign lesions usually have doubling times of fewer than 30 days or greater than 450 days, and malignant lesions have doubling times between these values.77.78.79.80. and 81.
Given the ability to obtain nodule volumes using computer-assisted evaluation, the evaluation of nodule growth on CT examinations has received widespread attention. Numerous software programs are now available that allow nodule segmentation and volume calculation. Several studies have shown greater accuracy of CT-derived volumetric measurements confined to subjective estimations, mainly because subtle volume changes may not be visually detectable and because growth in the craniocaudal direction is difficult to assess on transverse CT sections.82.83. and 84. Although computer-aided analysis of nodule volume is likely to gain importance in the future, 82 diagnostic evaluation remains largely based on visual identification of a change in cross-sectional diameter. 85
Central tumors
The cardinal imaging signs of a central tumor are collapse and consolidation of the lung beyond the tumor and the presence of hilar enlargement, signs that may be seen in isolation or in conjunction with one another.
Collapse and consolidation in association with central tumors
Obstruction of a major bronchus may lead to a combination of atelectasis and retention of secretions with consequent pulmonary opacity, 86 but collateral air drift may partially or completely prevent these postobstructive changes. Obstructed portions of the lung may become secondarily infected, though this is relatively uncommon, at least in patients whose tumors are still amenable to surgical resection. 86 With time, lipid-laden alveolar macrophages accumulate within the airspaces distal to an obstruction, giving rise to an appearance known to pathologists as endogenous lipoid pneumonia (‘golden pneumonia’). The interstitium thickens because of chronic inflammatory infiltration and collagen deposition. 86 As might be expected, the cancer most frequently responsible for collapse and consolidation is squamous cell carcinoma, partly because it is a common cell type and partly because a larger proportion of squamous carcinomas originate centrally.
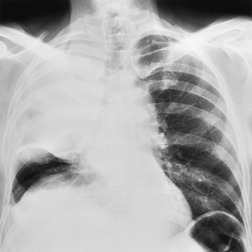
Collapse and consolidation beyond an obstructing lung cancer are readily recognizable radiographically as patchy (Fig. 13.12) or homogeneous pulmonary opacity. Loss of volume is usual with central tumors, but consolidation without loss of volume may be encountered. Air bronchograms visible on chest radiographs are uncommon, particularly before antibiotic therapy, but they are seen fairly frequently at CT (Fig. 13.13). If the tumor regresses with therapy, a previously invisible air bronchogram may become visible on chest radiographs.
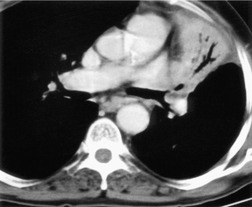 |
| Fig. 13.13 |
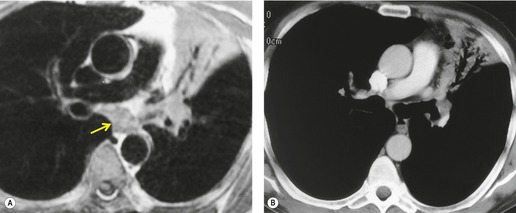
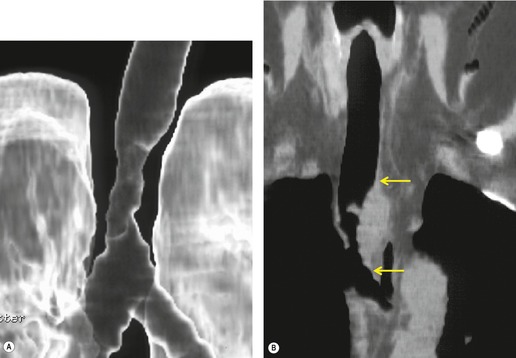
Retained mucus may accumulate in and distend the airways. Mucus-filled dilated bronchi are more apparent on the postcontrast CT than they are on the images taken prior to contrast administration, 87 and when seen should prompt a search for a centrally obstructing tumor. They may be seen within collapsed lobes on CT or MRI examinations as branching tubular structures (Fig. 13.14) or, when seen in cross-section, as round or oval densities.
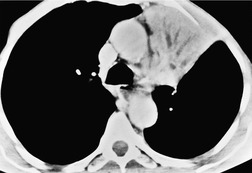 |
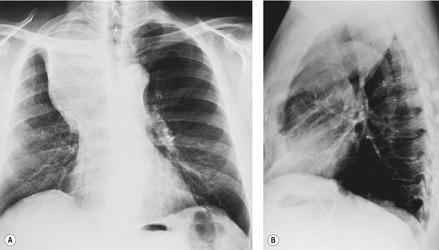
| Fig. 13.14 Dilated fluid-filled bronchi in a collapsed left upper lobe beyond a central bronchial carcinoma. (Chest radiograph of this patient is shown in Fig. 13.16.) |
Defining the presence or extent of the central tumor mass in the presence of postobstructive consolidation and atelectasis can be difficult. This decision can be important in making the initial diagnosis of a central tumor. It is also an important factor in planning radical radiotherapy. Although CT or MRI may allow the tumor to be more accurately measured, the size of the lesion does in itself affect decisions regarding surgery, since both small and large tumors are surgically excised, provided they have not spread too far. On unenhanced CT, the neoplastic and non-neoplastic tissue may be similar in density. After administration of contrast material there is differential enhancement, most obvious if rapid scanning is used: the neoplastic tissue enhances to a minimal degree whereas distal atelectasis may show substantial enhancement. 88 MRI shows a difference in signal between tumor and postobstructive pulmonary changes, particularly on T2-weighted images or gadolinium-enhanced T1-weighted images.89. and 90. Based on one small series it appears that cholesterol pneumonitis and distal bronchiectasis are seen as higher signal than tumor on T2-weighted images, whereas organizing pneumonia and atelectasis are isointense with tumors. 91
The following features suggest that pneumonia is secondary to an obstructing neoplasm (summarized in Box 13.3):
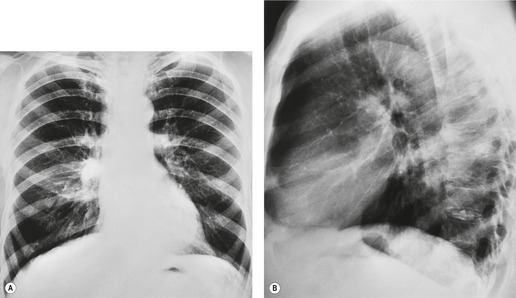
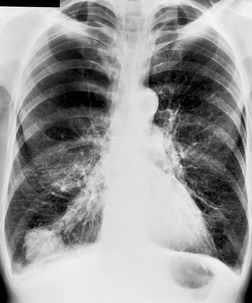
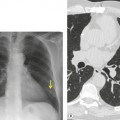
• Alteration in the shape of the collapsed or consolidated lobe due directly to the bulk of the underlying tumor. The fissure in the region of the mass may be unable to move in the usual manner, with the result that the fissure appears bulged (‘Golden S’ sign) (Fig. 13.15). This sign indicates that the collapse is the result of an underlying mass and predicts that the mass will be sufficiently central that successful bronchoscopic biopsy should be readily achievable.
• The presence of pneumonia confined to one lobe (or more lobes if there is a common bronchus supplying multiple lobes) in patients over the age of 45 years, particularly if the lobe shows loss of volume and no air bronchograms (Fig. 13.16). Occasionally the opacified lobe appears larger than normal because of secretions and infection trapped behind the obstructing carcinoma (Fig. 13.17), an appearance that has been labeled the ‘drowned lobe’. In cases of obstructive pneumonitis or atelectasis the tumor should be readily visible at bronchoscopy, an investigation that is usually performed without delay.
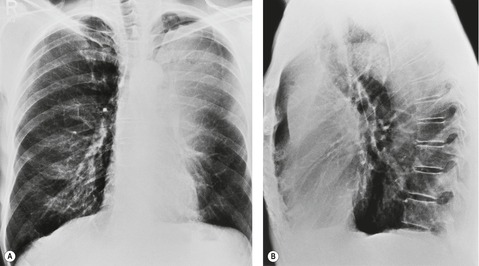 |
| Fig. 13.16 Left upper lobe collapse beyond a centrally obstructing lung cancer shown on A, posteroanterior and B, lateral chest radiograph. (CT of this patient is shown in Fig. 13.14.) |
• The presence of a visible mass or irregular stenosis in a main stem or lobar bronchus. Careful analysis of CT images will demonstrate the presence of an obstructing tumor in virtually every case of postobstructive atelectasis caused by a lung cancer. 92
• The presence of an associated central mass. Simple pneumonia rarely causes radiographically visible hilar adenopathy, although enlarged central nodes may be seen on CT or MRI. Bacterial lung abscess can, however, be confused with lung cancer because it may result in hilar or mediastinal adenopathy.
• A localized pneumonia that persists unchanged for more than 2 weeks or one that recurs in the same lobe. Simple pneumonia often clears or spreads to other segments during this time interval. Complete resolution of pneumonia, in practice, excludes an obstructing neoplasm as the cause of infection. Although consolidation may improve partially with appropriate antibiotic therapy, it virtually never resolves completely if it is secondary to an underlying carcinoma.
• Visibly dilated fluid-filled bronchi in the affected lobe on CT or MRI.
Box 13.3
• Golden S sign
• Pneumonia confined to one lobe (or more lobes if supplied by a common bronchus)
• Expansion of a consolidated lobe
• Visible stenosis of supplying bronchus
• Visible central mass
• Localized pneumonia that is unchanged for more than 2 weeks or one that recurs in the same lobe after a short interval
• Visibly dilated fluid-filled bronchi at CT or MRI
Hilar enlargement
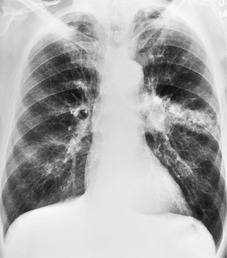
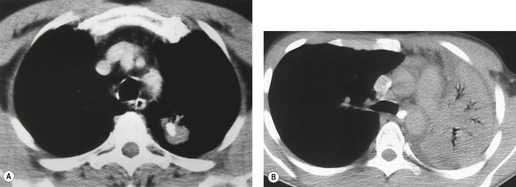
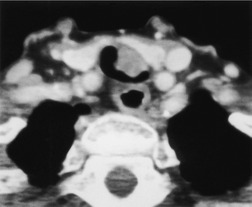
Hilar enlargement is a common presenting feature on chest radiographs in patients with lung cancer. In the Mayo Clinic series, 38% of patients had a hilar or perihilar mass, and in 12% a central mass was the only radiographic abnormality.24.49.93. and 94. Such masses may result from the tumor itself, from enlargement of hilar nodes containing metastatic tumor, from consolidated lung, or from a combination of these phenomena. Deciding their relative contribution to the tumor mass can be difficult on chest radiographs, and indeed on CT (Fig. 13.18). In general, the more lobular the shape, the more likely it is that enlarged lymph nodes are present.
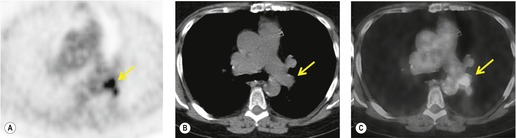 |
| Fig. 13.18 (Courtesy of John Anthony Parker, Boston, MA, USA.) |
A mass superimposed on the hilum may lead to increased density of the hilum because of summation of the opacity of the mass added to the density of the normal hilar opacity (Fig. 13.19). Increased hilar density may be the only indication of lung cancer on a frontal chest radiograph; when the sign is suspected, careful inspection of a lateral radiograph or CT is essential.
Staging intrathoracic spread of tumor
The international staging system
For staging purposes, lung carcinoma is classified into nonsmall cell and small cell types, reflecting the marked differences in natural history and response to therapy.
The International Staging System for nonsmall cell lung cancer stratifies disease extent in terms of prognosis, and has been adopted by the International Union Against Cancer. 95 It is based on the TNM grading of the primary tumor, regional nodes, and distant metastases (Table 13.2). The stages have been devised to produce groups (see Tables 13.3 and 13.4) which reflect the management options and survival (see Table 13.5). Refinements of the T and M descriptors, as well as the tumor stage groupings, are expected for the seventh edition of the TNM Classification of Malignant Tumors in the course of 2009 (Box 13.4).96. and 97.
| Primary tumor (T) | |
| T1 | Tumor ≤3 cm diameter, surrounded by lung or visceral pleura, without invasion more proximal than lobar bronchus |
| T1a | Tumor ≤2 cm in diameter |
| T1b | Tumor >2 cm in diameter |
| T2 | Tumor >3 cm but ≤7 cm, or tumor with any of the following features: |
| Involves main bronchus, ≥2 cm distal to carina | |
| Invades visceral pleura | |
| Associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung | |
| T2a | Tumor ≤5 cm |
| T2b | Tumor >5 cm |
| T3 | Tumor >7 cm or any of the following: |
| Directly invades any of the following: chest wall, diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium, main bronchus <2 cm from the carina (without involvement of the carina) | |
| Atelectasis or obstructive pneumonitis of the entire lung | |
| Separate tumor nodules in the same lobe | |
| T4 | Tumor of any size that invades the mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina, or with separate tumor nodules in a different ipsilateral lobe |
| Regional lymph nodes (N) | |
| N0 | No regional lymph node metastases |
| N1 | Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension |
| N2 | Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s) |
| N3 | Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s) |
| Distant metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Separate tumor nodule(s) in a contralateral lobe; tumor with pleural nodules or malignant pleural or pericardial effusion |
| M1b | Distant metastasis |
| Stage | Definition |
|---|---|
| Stage IA | T1a–T1b N0 M0 |
| Stage IB | T2a N0 M0 |
| Stage IIA | T1a–T2a N1 M0 |
| T2b N0 M0 | |
| Stage IIB | T2b N1 M0 |
| T3 N0 M0 | |
| Stage IIIA | T1a–T3 N2 M0 |
| T3 N1 M0 | |
| T4 N0–N1 M0 | |
| Stage IIIB | T4 N2 M0 |
| T1a–T4 N3 M0 | |
| Stage IV | Any T Any N M1a or M1b |
| Stage I | No nodal metastases and totally removable by lobectomy or pneumonectomy. Divided into A or B based on tumor size/involvement of major bronchi |
| Stage II | Adds hilar node involvement or T3 tumor with no node involvement |
| Stage IIIA | Extensive but resectable disease (T3 N1, T1 N2, T2 N2, T3 N2) |
| Stage IIIB | Irresectable disease by conventional criteria but still confined to chest, so eligible for radical radiotherapy |
| Stage IV | Distant metastases |
| Stage | Clinical staging | Pathological staging | ||
|---|---|---|---|---|
| USA99 | Japan100 | USA (%) 99 | Japan (%) 100 | |
| IA | 61 | 71 | 67 | 79 |
| IB | 38 | 44 | 57 | 60 |
| IIA | 34 | 41 | 55 | 57 |
| IIB | 24 | 37 | 39 | 45 |
| IIIA | 13 | 23 | 23 | 24 |
| IIIB | 5 | 20 | – | 16 |
| IV | 1 | 22 | – | 5 |
Box 13.4
Primary tumor (T)
• T1 lesions are divided based on size into T1a (≤2 cm) and T1b (>2 cm but <3 cm)
• T2 lesions are divided into T2a (≤5 cm) and T2b (>5 cm but ≤7 cm)
• T2 tumors >7 cm are reclassified as T3
• T4 tumors with satellite nodules in the same lobe as the primary tumor are reclassified as T3
• Additional nodules in a different lobe of the same lung are reclassified as T4 rather than M1
• Malignant pleural or pericardial effusions or pleural nodules are now classified as metastasis (M1a) rather than T4
Regional nodes (N)
• No changes
Metastasis (M)
• Subdivided into M1a (malignant pleural or pericardial effusion, pleural nodules, nodules in contralateral lung) and M1b (distant metastasis)
Stage grouping
• T2a N1 M0 lesions are classified as IIA, rather than IIB
• T2b N0 M0 lesions are classified as IIA, rather than IB
• T3 (>7 cm) N0 M0 lesions are classified as IIB, rather than IB
• T3 (>7 cm) N1 M0 lesions are classified as IIIA, rather than IIB
• T3 N0 M0 (nodules in same lobe) lesions are classified as IIB, rather than IIIB
• T3 N1 M0 or T3 N2 M0 (nodules in same lobe) are classified as IIIA, rather than IIIB
• T4 M0 (ipsilateral lung nodules) lesions are classified as IIIA (if N0 or N1) and IIIB (if N2 or N3), rather than stage IV
• T4 M0 (direct extension) lesions are classified as IIIA (if N0 or N1), rather than IIIB
• Malignant pleural effusions (M1a) are classified as IV, rather than IIIB
Small cell lung cancer is classified as limited (confined to one hemithorax but may involve contralateral mediastinal and supraclavicular nodes) or extensive, according to the requirements for radiotherapy fields. 98 SCLC is generally regarded as a systemic disease and is usually disseminated from the outset. The major role for imaging is to determine extrathoracic spread, a topic beyond the scope of this discussion.
As with most other cancers, the treatment options and outcomes for lung cancer are heavily dependent on the stage and cell type (see Table 13.5).
The treatment of choice for nonsmall cell lung cancer, in the absence of evidence of dissemination, is surgical resection. The prime issue for the surgeon, therefore, is whether the tumor can be completely removed by surgery. Clear surgical margins in resection specimens and absence of tumor cells in resected lymph nodes are prime determinants of local recurrence and survival. 101
Stage I and II tumors are treated by lobectomy or pneumonectomy, 102 with selected stage II tumors receiving adjuvant radiotherapy and/or chemotherapy. 103 Tumors that extend along the main bronchi to within 2 cm of the carina, but do not involve it, are included in stage II provided there is no nodal involvement. Such tumors may be resected with bronchoplastic techniques. 104 T3 tumors are regarded as operable provided there is no nodal or distant spread (i.e. still stage II), but the results of surgery are less good than for T1 and T2 tumors, even when complete clearance of tumor is possible.
The treatment of stage IIIA tumors ranges from surgical resection to nonsurgical modes of treatment and varies greatly from center to center. 105 Some patients may benefit from mediastinal lymphadenectomy of involved ipsilateral nodes (N2). Stage IIIB tumors involve critical mediastinal structures such as the great vessels, esophagus, and trachea (T4), or have spread to contralateral mediastinal nodes (N3). Patients with stage IIIB tumors are not considered to be surgical candidates, unless preoperative neoadjuvant chemotherapy is given to ‘downstage’ the tumor.99. and 106.
In patients with NSCLCs deemed unsuitable for surgery, due to either intrathoracic spread or metastatic disease, treatment options include various chemotherapy and radiotherapy regimens, which in suitable patients in some centers may be followed by surgical resection. Radical radiation therapy requires the tumor volume to be encompassed within a suitable radiation field, in a manner that critical organ and total body doses are not exceeded. The oncologist needs to know the total disease burden, and disease quantification is also important when measuring therapeutic response. In addition, imaging can help to guide biopsy and can confirm and characterize metastases.
Imaging for staging nonsmall cell lung cancer
Currently, the standard imaging techniques used to stage the intrathoracic spread of lung cancer are chest radiography and CT.31. and 110. In some centers bronchoscopy is undertaken prior to CT, but in other centers CT is done routinely before bronchoscopy, an approach that can be justified on the grounds that it is cost-effective and, on occasion, will obviate the need for bronchoscopy by showing irresectable disease, or by showing benign disease only. 111
CT is usually performed following intravenous contrast enhancement, but the evidence for the routine use of contrast is relatively weak. In a series of 96 patients with pathologically proven lung cancer, no change in management resulted from the availability of contrast-enhanced CT of the chest and liver compared with unenhanced CT of the chest down to the adrenal glands. 112 Another study investigated whether more enlarged mediastinal nodes were evident on post- as opposed to noncontrast-enhanced examinations113 and found that only in station 2R were significantly more nodes appreciated following contrast. The detection of hilar lymph nodes using CT is, however, significantly better following contrast enhancement. 114
It should be appreciated that even CT, which is significantly more sensitive than chest radiographs, disagrees with the TNM stage found at surgery in a significant proportion of patients.88. and 115. In 40% of cases in one typical series CT categorized the extent of tumor sufficiently poorly that the overall stage was overestimated or underestimated. 116
The preoperative decision whether lobectomy or pneumonectomy will be required for centrally situated tumors, or whether conservative bronchoplastic surgery, i.e. sleeve lobectomy or pneumonectomy, 117 will be feasible, depends on whether or not the tumor has crossed fissures, invaded central vessels, or spread centrally within the bronchial tree. Chest radiography and CT, particularly multiplanar CT using multidetector scanners, and virtual CT bronchoscopy provide important information, but have not, in general, proved sufficiently accurate in predicting whether or not a pneumonectomy will be required. Currently, therefore, the surgeon still mostly makes this decision based on bronchoscopic findings or on the findings at thoracotomy.115. and 118.
Even with tumors amenable to surgical resection, a major decision in many patients is whether or not lung function will remain adequate once a pneumonectomy has been carried out. Pulmonary perfusion scans have a role here. The relative perfusion of each lung can be quantified from the number of radioactive counts in the combined anterior and posterior scans. The percentage contribution of each lung is then multiplied by the overall forced expiratory volume in 1 second (FEV1) to predict the FEV1 of the lung that would remain after surgery. 119 Quantitative regional ventilation and perfusion can also be assessed using nuclear medicine techniques in order to predict postoperative loss of lung function. 120 Recently, dynamic perfusion MRI has been shown able to predict postoperative lung function in patients after lung cancer resection. 121
Staging the primary tumor
T1 and T2 tumors are confined to the lung and its investing pleura, or to bronchi more than 2 cm from the carina. T3 tumors have limited extrapulmonary extension, including invasion of the chest wall, mediastinal pleura, pericardium, diaphragm, and thoracic apex, but are mostly considered to be resectable. Tumors that extend to within 2 cm of the carina, but do not involve the carina are also classified as T3. T4 tumors invade the heart, great vessels, trachea, carina, esophagus, or vertebral body. Any tumor associated with a malignant pleural effusion is also designated T4.
The distinction between T3 and T4 tumors is critical because it reflects the dividing line between conventional surgical and nonsurgical management. T4 tumors make the disease at least stage IIIB and are regarded as irresectable by the great majority of surgeons, either because they have invaded the vertebrae or critical mediastinal structures, such as the heart and great vessels, trachea and carina, and esophagus, or because they have disseminated to the pleura or within a lobe. A few surgeons try to resect tumors in the occasional highly selected patient with a T4 tumor, provided complete resection can be performed. 122 Some of the patients will have had a preoperative course of neoadjuvant chemotherapy in order to ‘downstage’ the tumor.123. and 124. There are anecdotal reports of 5-year survivors who have undergone reconstruction of the superior vena cava, resection of vertebral bodies, or partial cardiac dissections.125. and 126. However, most surgeons believe that such radical surgery is not justified. (Invasion of the diaphragm is classified as T3, but deep invasion of the diaphragm was shown in one large surgical series to be associated with an outcome similar to T4 tumors. 127)
It is easy to assess the size and position of a primary tumor surrounded by aerated lung on chest radiographs and with CT. It may, however, be difficult to distinguish the tumor from distal collapsed or consolidated lung on CT and, therefore, overestimate or underestimate tumor size and extent of chest wall or mediastinal contact. At contrast-enhanced CT, collapsed lung enhances more than central tumor, 128 and may show mucus-filled bronchi, an indicator of collapsed lung. T2-weighted MRI can be useful for separately identifying tumor from distal collapse/consolidation. 129 The tumor usually shows much lower T2 signal than the distal changes, and mucus-filled dilated bronchi can be specifically identified as high-intensity tubular structures. Despite its overall importance in cancer staging, PET scanning has not proved to be of use in determining the extent of the primary tumor. 130
Mediastinal invasion
The chest radiograph is a poor indicator of mediastinal invasion, although involvement of the phrenic nerve is suggested by elevation of the ipsilateral hemidiaphragm, particularly if it is a new finding. Caution is needed before deciding that a high hemidiaphragm is caused by phrenic nerve invasion, because lobar collapse can lead to diaphragm elevation, and subpulmonary effusion may mimic it. Ultrasonography can provide information about diaphragmatic movement and, by inference, phrenic nerve involvement. 131
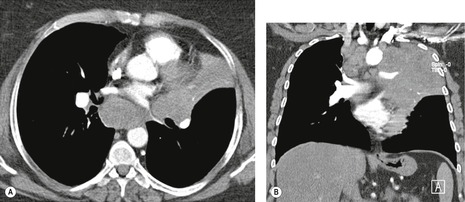
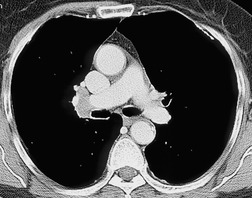
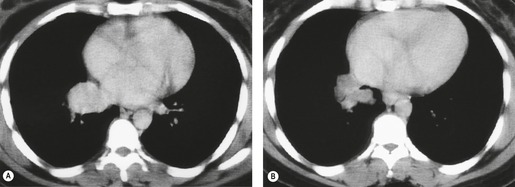
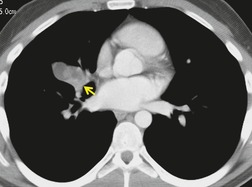
Both CT and MRI (see Table 13.4) can show the presence of extensive tumor within the mediastinum. Clear-cut encasement of vital structures such as the esophagus, trachea, or major vessels, or deep penetration of tissue planes, is conclusive evidence of a T4 tumor (Figs 13.20 and 13.21). Mere contact with the mediastinum is not enough for the diagnosis of invasion (Figs 13.21CD, 13.22), and apparent interdigitation with mediastinal fat can be a misleading sign on both CT and MRI. Also, associated pneumonia or atelectasis may make it difficult to determine whether mediastinal contact is even present (Fig. 13.23).
Multidetector CT has enabled better assessment of mediastinal invasion. 88 Advantages include more reliable contrast opacification of vascular structures, reduced cardiac and respiratory motion artifact, and limitation of partial volume averaging. High-quality multiplanar reformations allow detailed assessment of important regions such as the tracheal carina, aortopulmonary window, and aortic arch. Visualization of the bronchial tree using planar and three-dimensional (3D) techniques is now an established technique.
• The CT features of limited mediastinal contact or preserved mediastinal fat plane (<3 cm, <90 degrees of circumferential contact with descending aorta) are reasonably accurate at predicting tumor resectability
• MRI is of similar value; its multiplanar capability is advantageous only in specific regions
• Both techniques are less accurate at identifying T4 disease and irresectability
Some groups have investigated specialized CT techniques in an attempt to obtain more accurate information. Ultrafast cine CT with respiratory and cardiac gating has been used to show relative movement between the tumor and mediastinal structures, implying lack of invasion.132. and 133. CT performed following the induction of a diagnostic pneumothorax can similarly reveal lack of tumor fixation. 134 Although these techniques are effective methods of excluding mediastinal extension in small series, they do not address the important converse problem, namely diagnosing invasion, because benign adhesions, as well as tumor invasion, can cause fixation of lung to the mediastinum.
In certain specific situations, MRI may be superior to single detector CT for the assessment of mediastinal invasion, but the case for the routine use of MRI for the diagnosis of mediastinal invasion has not been made; the signs are basically the same and the axial imaging plane is the standard projection for both tests. MRI is no more accurate than CT in distinguishing between contiguity of tumor with the mediastinum and mediastinal invasion, largely because invasion of the mediastinal fat can be mimicked by adjacent inflammatory changes.90.135. and 136.
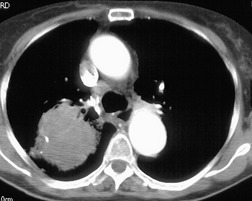
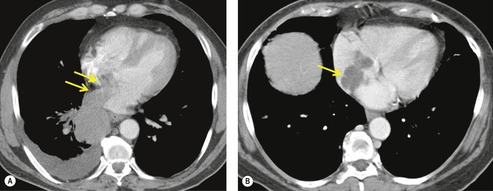
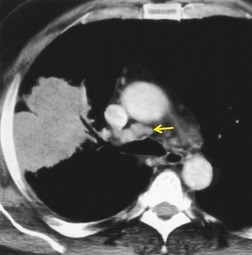
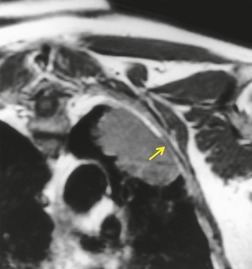
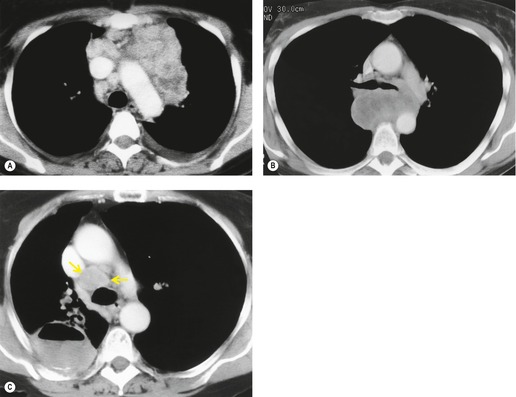
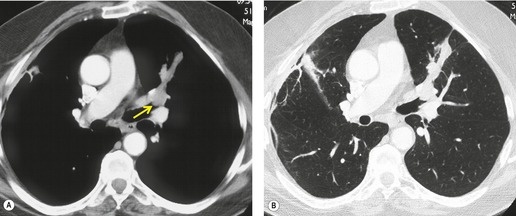
MRI can, however, provide useful information in certain circumstances. 129 Endobronchial tumor extension remains the province of bronchoscopy, but extraluminal encasement is well seen by MRI. Also, the ability to show the superior vena cava in the coronal plane without having to inject contrast medium allows easy, often exquisite, demonstration of the extent of tumor in patients with superior vena caval syndrome. Similarly pulmonary vein, pericardial, and cardiac involvement are well demonstrated. 137 Tumor may grow along the pulmonary veins to become intrapericardial or to lie within the left atrium – features that can be well demonstrated by MRI or contrast-enhanced CT (Fig. 13.24). The normal pericardium is seen as a low signal membrane and disruption of the pericardium can, therefore, be recognized. Multiple ECG-triggered contrast-enhanced magnetic resonance (MR) angiography allowed better detection of hilar and mediastinal invasion than nongated MR angiography in one series. 138
Chest wall invasion
A peripheral lung tumor may cross the parietal pleura and invade ribs and intercostal muscles. Such localized invasion of the chest wall (T3) by NSCLC need not be a contraindication to surgery, but it adversely affects prognosis and alters the surgical technique, requiring en bloc resection and, in some instances, chest wall reconstruction.139. and 140. Five-year survivals of up to 40% have been achieved, provided all microscopic tumor has been cleared. The main determinant of outcome is the existence of hilar/mediastinal nodal disease, rather than the presence or depth of chest wall invasion. The value of any imaging assessment is to predict the extent of surgery required. The value of imaging chest wall invasion depends on the surgeon’s practice. 141 Provided the surgeon is prepared to perform a chest wall resection, the surgical technique can be modified according to the operative findings.
The chest radiograph can reveal advanced rib or spinal destruction (Fig. 13.25A) but does not enable minor degrees of chest wall invasion to be diagnosed.
Several CT signs of parietal pleural invasion have been described, including an obtuse angle of contact between the tumor and the chest wall, obliteration of the extrapleural fat plane, pleural thickening, and the presence of extrapleural soft tissue. Tumor tissue clearly destroying bone (Fig 13.25B) or extending through the intercostal muscles beyond the line of the ribs is undoubted evidence of invasion, but lesser degrees of involvement are harder to evaluate. Contact with the pleura (Fig. 13.26), even if the pleura is thickened, does not necessarily indicate invasion, and increased density of the extrapleural fat adjacent to a lung tumor can be due to inflammatory reaction rather than neoplastic invasion (see Fig. 13.35). However, the greater the degree of contact and the greater the pleural thickening, the more likely it is that the parietal pleura has been invaded, particularly if the extrapleural fat plane is obliterated. A clear extrapleural fat plane adjacent to the mass may be helpful, but again not definitive, in excluding chest wall invasion (Fig. 13.27). Local chest wall pain may still be the most specific indicator of invasion.142.143.144.145. and 146.
Modified CT techniques have been studied. Diagnostically induced pneumothoraces can reveal separation of a pulmonary mass from the chest wall and so exclude invasion.134. and 147. CT performed during the respiratory cycle may reveal relative movement between the tumor and chest wall, implying lack of fixation. 132 Multiplanar reformations may help assess lesions adjacent to the diaphragm. One recent study showed that 3D surface-shaded reformations could characterize pleural puckering, and distinguish visceral from parietal pleural invasion with 80% accuracy. 148
On MRI, tumor tissue beyond the line of the ribs, as with CT, is good evidence of chest wall invasion (Fig. 13.28). A key observation is the appearance of the extrapleural fat, which is seen best as a thin high-intensity line on T1-weighted images. 149 In one prospective study of 34 patients, the presence of material of identical signal to tumor extending into the layer of fat was 85% sensitive and 100% specific for chest wall invasion, 149 but other series did not provide such good results. Dynamic cine MRI during breathing can, like CT, demonstrate lack of fixity of a tumor to the adjacent chest wall and so exclude parietal pleural and chest wall involvement, but cannot distinguish between benign adhesions and malignant tumor crossing the pleural cavity.150. and 151.
Apical tumors (Pancoast, superior sulcus tumors)
Apical, or Pancoast, tumors deserve special mention. The eponym nowadays refers to the symptom complex of pain in the shoulder and arm that results from an apical tumor invading the lower cords of the brachial plexus and the sympathetic chain. Pancoast’s original description included ipsilateral Horner syndrome from invasion of the sympathetic chain and local destruction of bone by the tumor. 152 Because these tumors arise adjacent to the groove for the subclavian artery, they are also called superior sulcus tumors. They may be of any cell type, 153 and have a propensity to invade the adjacent chest wall, root of neck, brachial plexus, subclavian vessels, and spine. In some centers survival has been significantly increased by preoperative radiation therapy and en bloc resection of the tumor, providing the tumor is technically resectable. In such centers, preoperative CT and MRI are essential. 122
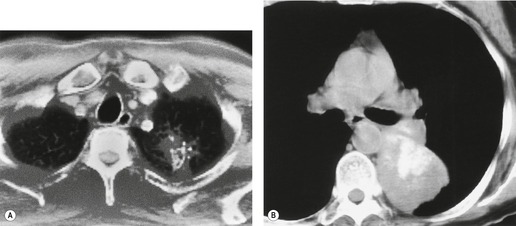
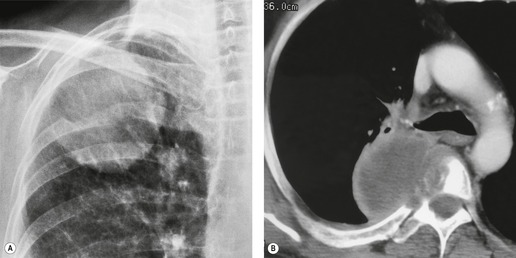
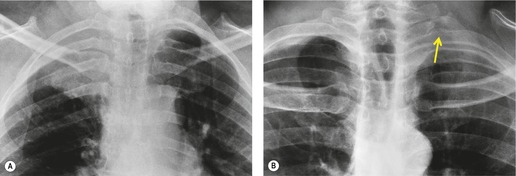
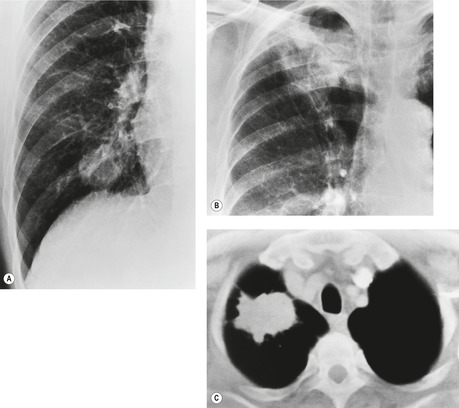
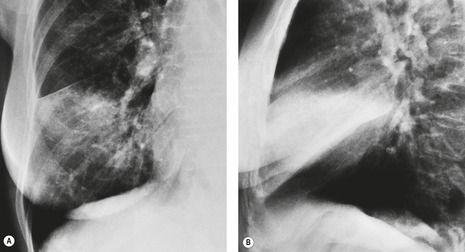
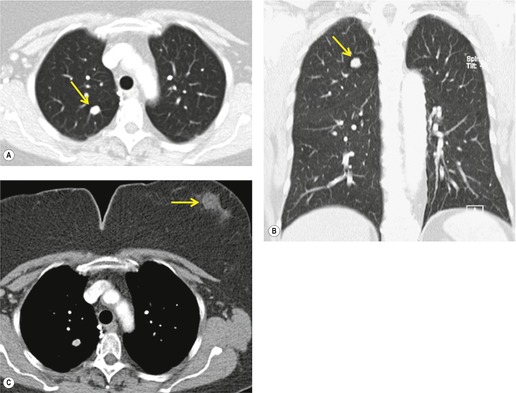
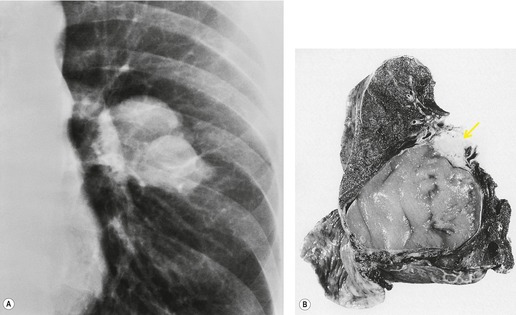
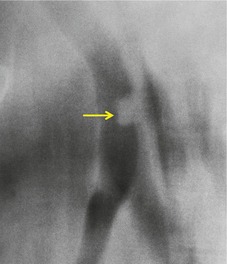
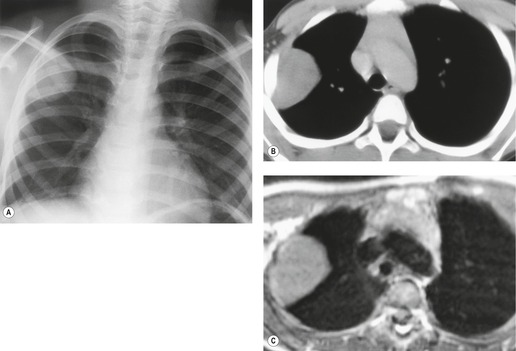
Radiographically, 154 superior sulcus tumors appear as a mass in approximately one-half to three-quarters of cases and as an apical cap resembling pleural thickening in the remainder. Bone destruction of the adjacent ribs or spine is seen on chest radiographs in approximately one-third of cases (Fig. 13.29). 155 These tumors are, however, often difficult to diagnose on chest radiographs because the lung apex is partly hidden by overlying ribs and clavicles. Also, the tumor so often closely resembles a benign apical pleural cap (Figs 13.29 and 13.30) and the cardinal plain film sign of the lesion – bone destruction – is either absent or difficult to diagnose with confidence. Asymmetric pleural thickening, particularly if associated with appropriate symptoms, should be viewed with suspicion. A chronically enlarging unilateral apical cap strongly suggests superior sulcus carcinoma. 156
CT can be helpful for diagnosing Pancoast tumors (Fig. 13.31).157. and 158. It may demonstrate an intrapulmonary mass rather than just pleural thickening, providing extra confidence that the diagnosis is neoplasm rather than inflammatory pleural disease. Also, CT is a sensitive technique with which to diagnose the full extent of tumor, particularly any chest wall invasion.
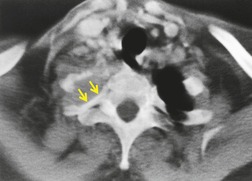 |
| Fig. 13.31 |
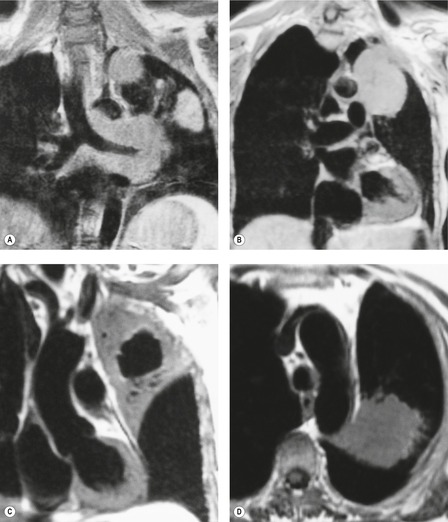
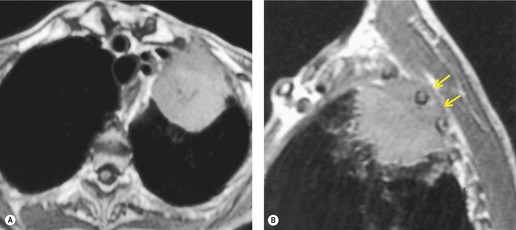
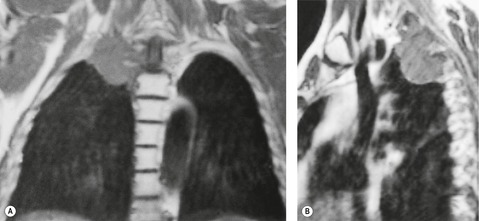
MRI is regarded as the optimal technique for demonstrating the extent of superior sulcus tumors, largely because the coronal and sagittal planes are the optimal imaging planes to demonstrate the cupola shape of the chest wall in the apical regions and to show the brachial plexus, subclavian vessels, neural foramina, and any bone marrow invasion to advantage (Fig. 13.32).129.159.160. and 161. Thin sections and surface coils are recommended. T1-weighted and STIR (short tau inversion recovery) images in coronal and sagittal planes appear to be the optimal sequences. Interruption of the normal extrapleural fat line over the lung apex can be readily shown, as can the relationship of tumor to brachial plexus, subclavian vessels, and spinal canal. Rib and vertebral body destruction, however, may be less well shown by MRI than by CT.
Intrathoracic lymph node staging (see Box 13.6)
The most important predictor of outcome in the majority of patients with NSCLC limited to the chest is the presence or absence of involved mediastinal lymph nodes. 162 Surgery is not a curative option for patients with positive contralateral mediastinal nodes (N3). Surgery is also considered inappropriate in symptomatic N2 disease. The surgical management of lesser degrees of N2 disease is controversial. Conflicting published results are partly due to differences in selection criteria and data analysis. Some surgeons operate with the hope of cure on patients with N2 disease as long as the involved nodes can be completely resected, are few in number, are not bulky, and are not in the high paratracheal region. 101 The prognosis correlates with the method by which N2 disease is established. Positive nodal disease, established only after operative mediastinal dissection, has a better outcome than nodal involvement discovered preoperatively by mediastinal biopsy or CT. Several recent series have confirmed that a 20–32% 5-year survival can be achieved in patients discovered to have N2 disease at operation after negative preoperative CT or mediastinoscopy, provided that complete removal of tumor is possible.163.164.165. and 166. In one series, a 5-year survival of 57% was achieved in a subset of patients with N2 disease in whom the nodes were not enlarged by CT criteria and in whom only single level nodes, which could be completely removed at surgery, were involved. 165 Some authors noted more favorable outcomes with squamous carcinoma than with other cell types, 101 but most have not found the cell type for NSCLCs to be a remarkable factor.
Box 13.6
• CT can display enlarged mediastinal nodes, but its accuracy for diagnosing metastatic disease is limited by the lack of specificity of nodal enlargement
• In general, MRI currently has little advantage over CT for imaging mediastinal nodes
• Radionuclide studies, particularly (FDG)-PET and PET-CT, have evolved into important techniques in the assessment of lymph node involvement
• In certain situations endosonography can identify metastatic mediastinal nodes and allow sampling
• In cases of inconclusive PET or PET-CT examinations, patients with enlarged mediastinal nodes should undergo a targeted biopsy before being denied the chance of surgical cure
Mediastinal nodal metastases are often present at the time of initial diagnosis of NSCLCs, particularly with adenocarcinomas167and small cell tumors. Primary tumors greater than 3 cm in diameter (T2 tumors) have a higher incidence of mediastinal nodal involvement than tumors of smaller diameter. 167 Also, the more central the primary tumor, the more likely it is to be accompanied by nodal metastases.
The position of hilar and mediastinal nodes should be described according to the recently unified American Thoracic Society (ATS) and American Joint Committee on Cancer (AJCC) classification as summarized by Mountain and Dresler,168.169. and 170. which uses fixed anatomic landmarks to localize individual nodal stations. It is worth noting that there is no division into right and left subcarinal stations, so subcarinal nodal disease is always designated N2.
Distinction between nodal stations may be difficult and subject to interobserver variation. 171 The pleural boundaries cannot always be resolved at CT, and even at surgery their location varies with the force of retraction. 172 The differentiation between hilar nodes (station 10), which lie outside the mediastinal pleural reflection, and the adjacent tracheobronchial nodes (station 4, e.g. azygos nodes) or subcarinal nodes (station 7), which are contained within the mediastinal pleura, can therefore be problematic, particularly on the right. 163 The differentiation between these locations is, however, of crucial importance because tracheobronchial or subcarinal node involvement converts N1 to N2 disease. 173 This factor may also influence the interpretation of data on the accuracy of imaging techniques.
Spread is usually sequential, first to the ipsilateral segmental, interlobar, or lobar intrapulmonary nodes (N1 nodes), then to ipsilateral hilar nodes (N1 nodes), and thereafter to ipsilateral mediastinal nodes (N2), but skip metastases to mediastinal nodes, with clear hilar nodes, are recognized in up to 33% of cases,174. and 175. and skip involvement of contralateral mediastinal nodes (N3) is not infrequent.
CT and MRI staging of nodal metastases
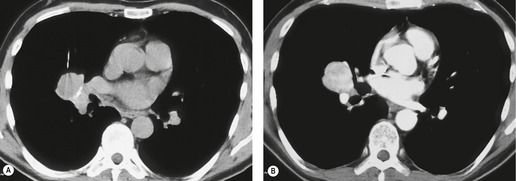
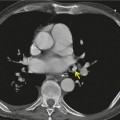
The most extensively validated CT sign of lymph node metastasis is nodal enlargement (Fig. 13.33). CT enhancement characteristics have proved to be unhelpful and low-density necrotic areas within a node, a good sign of metastatic involvement and one that has proved useful in diagnosing metastases from head and neck tumors in cervical lymph nodes, has proved to be infrequent in mediastinal nodal involvement by lung cancer.
Normal hilar lymph node size has been documented by Remy-Jardin et al., 114 the normal range being up to 7 mm in short axis diameter. Normal nodes show a flat or concave interface with the adjacent lung, whereas nodes which are enlarged due to metastasis show a convex bulge at the pulmonary interface. 176 Metastasis to hilar lymph nodes (N1 disease) (Fig. 13.34), although conferring a poorer prognosis, does not preclude potentially curative surgical resection.
Normal mediastinal lymph node size at CT varies according to location within the mediastinum: normal nodes in the subcarinal and lower paratracheal regions can be up to 11 mm in short-axis diameter, with a few reaching 15 mm, whereas normal nodes in the upper paratracheal regions rarely exceed 7 mm. A simple and reasonably accurate rule is that mediastinal nodes smaller than 10 mm in short-axis diameter fall within the 95th percentile and should, therefore, be considered normal.
The problem with using size as the only criterion for malignant involvement is that intrathoracic lymph node enlargement has nonmalignant causes, including previous tuberculosis, histoplasmosis, pneumoconiosis, sarcoidosis, and, notably, reactive hyperplasia to the tumor or to associated pneumonia and atelectasis (Figs 13.26 and 13.35). False-positive enlargement of mediastinal lymph nodes is more common with centrally located and large primary tumors. 177 One-half to two-thirds of enlarged nodes draining postobstructive pneumonia and atelectasis are free of tumor. Indeed these nodes can be remarkably enlarged: in the series by McLoud et al., 178 37% of nodes between 2 cm and 4 cm were hyperplastic and did not contain metastases. Conversely, microscopic involvement by tumor can be present without causing enlargement of affected nodes. The frequency of this phenomenon varies greatly in different series ranging from 7% to 40%.179.180.181.182. and 183. It is perhaps worth noting that the frequency of metastatic involvement in normal-sized nodes is significantly higher with central adenocarcinomas than with central squamous cell carcinomas. Therefore, there is no single measurement above which all nodes can be assumed to be malignant and below which all nodes can be considered benign.
The reported sensitivity and specificity of CT for diagnosing mediastinal nodal metastases depends on the nodal diameter used to distinguish normal from abnormal and the thoroughness of mediastinal dissection to provide histologic correlation. Formal mediastinal lymphadenectomy may reveal up to twice the number of positive nodes discovered by limited preoperative sampling. 184 Early reports quoted values above 85% for sensitivity and specificity, but later studies using complete mediastinal lymphadenectomy and 10 mm short-axis diameter as the cut-off measurement for normality obtained figures of the order of 50–65% for both sensitivity and specificity.178.185.186.187.188. and 189. Similar sensitivities but better specificity figures have been obtained from Japan190 and some,191. and 192. but not all, 193 centers in Europe, probably because the prevalence of coincidental histoplasmosis and other fungal disease is much lower than in the USA.
One method of reducing the frequency of false-positive interpretations is to ensure that nodes draining the tumor are larger than nodes elsewhere in the mediastinum. By counting only enlarged nodes (>10 mm short-axis diameter) that were at least 5 mm greater in diameter than nodes in regions not draining the tumor, Buy et al. 191 were able to achieve a 95% positive predictive value for nodal metastatic disease.
The use of MRI for staging nodal disease relies, like CT, on recognizing nodal enlargement, but there are additional problems (Figs 13.36 and 13.37). The considerable overlap of the T1 and T2 relaxation times of benign and malignant lymph nodes prevents the use of unenhanced MR signal intensity for tissue characterization. 89 Intravenous gadolinium has not proved useful for distinguishing between chronic inflammation and neoplastic involvement. Contrast agents containing ultrasmall iron particles that are taken up by reticuloendothelial cells in lymph nodes are being investigated. 194 The concept is that nodes replaced by tumor show no signal change, whereas inflammatory nodes take up the agent and show reduced signal on T1-weighted images. Not all the inflammatory nodes in the mediastinum show signal loss, and there are false-negative results.
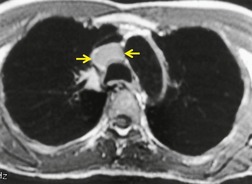 |
| Fig. 13.36 |
Radionuclide imaging for staging of nodal metastases
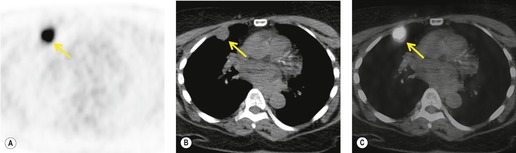
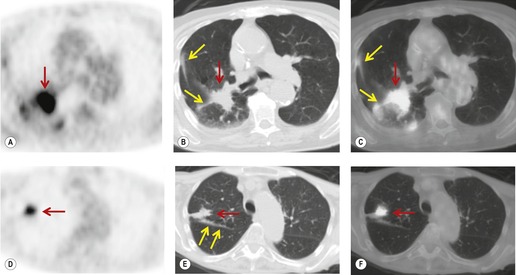
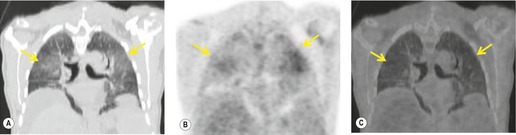
Radionuclide imaging techniques, particularly PET, are being used increasingly for staging lung carcinoma. FDG, a glucose analog with 18F substituted for one of the hydroxy groups, is the most widely used PET tracer. It is a marker for glucose metabolism which, after phosphorylation, is not metabolized further but remains trapped within tumor cells. FDG uptake is proportional to the metabolic rate of the cells which take up glucose and correlates with tumor aggressiveness and tumor growth rates.195. and 196. The specific criterion for a positive scan (Fig. 13.38) is either greater uptake in the lesion than in the background mediastinum, or a standardized uptake value of >2.5 (SUV is quantified as the ratio of the activity per estimated tumor volume compared with the activity administered to the patient, corrected for lean body mass). 197
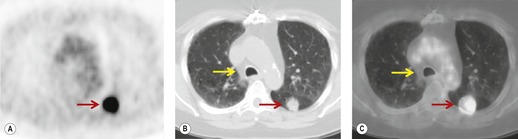 |
| Fig. 13.38 (Courtesy of John Anthony Parker, Boston, MA, USA.) |
The studies published so far have consistently shown significantly greater accuracy with PET than with CT for the detection or exclusion of mediastinal nodal disease,198.199.200.201.202.203.204.205.206.207.208.209.210.211. and 212. and PET has been shown to reduce the rate of futile thoracotomy213 and to influence patient management decisions.212. and 214. False-positive results are seen less frequently than with CT; the usual cause is inflammation of the lymph nodes due to incidental inflammatory disease or to reactive hyperplasia associated with pneumonia or atelectasis beyond the primary tumor. 215
In a metaanalysis of 514 patients who had undergone PET and 2226 patients who had undergone CT, the mean sensitivity of PET was 79% and the mean specificity was 91%, compared with a mean sensitivity for CT of 60% and a mean specificity of 77%. 216 FDG-PET imaging using a coincidence mode gamma camera has a significantly lower sensitivity and specificity than a dedicated PET scanner.217. and 218. Correlating PET and CT images improves the accuracy of FDG-PET compared with viewing the PET images in isolation. 207 For example, Vansteenkiste et al., 198 in a carefully conducted study, compared the accuracy of CT alone and FDG-PET plus CT for intrathoracic lymph node staging of 68 patients with potentially operable nonsmall cell lung cancer. The sensitivity of FDG-PET plus CT was 93% and the specificity was 95%. CT and PET image co-registration using computer techniques219 or collocation using combined CT and PET scanners, so called PET-CT machines, further increases the accuracy of PET scanning.220. and 221. Because of the excellent sensitivity of PET, it has been suggested that invasive mediastinal nodal staging can be substantially reduced when PET is negative (Fig. 13.38),222. and 223. particularly when both the PET and CT are normal.198. and 224. This recommendation is particularly strong for patients with presumed stage 1 disease. 225 False-positive PET images, due to infection, active inflammation, hyperplasia, etc., are sufficiently frequent to justify invasive staging in selected cases when PET is positive.
A decision analysis, using variables based on the literature, showed that a strategy whereby patients with enlarged nodes on chest CT or positive appearances on PET scanning undergo preoperative nodal biopsy whereas those in whom both the chest CT and the PET scan are normal go direct to thoracotomy can be cost-effective, compared with basing decisions on CT alone, without denying surgery to patients with resectable disease. The savings come from identifying inoperable patients prior to thoracotomy. A subsequent more detailed analysis from the same center confirmed the cost-effectiveness of adding PET to chest CT over a wide range of variables. 226
Endoscopic ultrasound for staging nodal metastases
In some centers, transesophageal ultrasound techniques are used to assess both the operability of the primary tumor and the presence of enlarged nodes.227.228. and 229. The technique is primarily of value in visualizing and sampling the right and left paratracheal (station 2R, 4L, and 4R) and subcarinal (station 7) nodes. The information regarding mediastinal lymph node metastases can be significantly more accurate than CT. Endosonographic features of neoplastic involvement of the nodes are rounded rather than oval shape, sharply demarcated border, and inhomogeneous hypoechoic texture, but the major value is to guide transesophageal fine-needle aspiration of visible nodes.
Spread to distant pulmonary sites
NSCLC can metastasize to the lungs. 230 The International Staging System classifies nodules of tumor in the same lobe as T4, whereas tumor nodules in another lobe on the ipsilateral side and all tumor nodules in the contralateral lung are classified as metastases, a distinction that is borne out by evidence that the prognosis of metastases to these different locations corresponds to T4 and M1, respectively. 231
The likelihood that pulmonary nodules detected by CT during staging for NSCLC are deposits of tumor is poorly quantified. Keogan et al. 232 showed that 16% of their 551 patients with potentially operable lung cancer had small noncalcified pulmonary nodules. Adequate follow-up was possible in only 25 patients and 70% of the nodules in these 25 patients proved to be benign. Kim et al. 233 found that 44% of 141 patients had small (10 mm diameter or less) nodules in lobes other than the lobe containing the primary carcinoma. Only six nodules in these 141 patients were malignant.
Pleural involvement
Lung carcinoma may involve the pleura by direct spread, lymphatic permeation, or tumor emboli. Visceral pleural invasion carries deleterious prognostic implications compared with tumors that do not invade the pleura. 234 This fact is recognized in the staging system by classifying all tumors that invade the visceral pleura as T2, even those below 3 cm in diameter. A study from Japan found that patients with tumors crossing a fissure to invade the adjacent lobe have the same survival as those with T3 tumors. 235
Pleural effusions occur with lung carcinoma of all cell types, but appear to be most frequent with adenocarcinoma. They may be freely mobile or may be loculated. Pleural effusion in association with a primary lung cancer designates the tumor as T4 except in the few patients who have clinical evidence of another cause for the effusion (such as heart failure) and in whom multiple pleural fluid cytologic examinations do not show tumor cells, in which case the effusion can be disregarded as a staging element. The presence of pleural effusion sufficiently large to be recognized at the time of diagnosis on chest radiographs in patients with lung cancer carries a poor prognosis, however, whether or not malignant cells are identified. 236
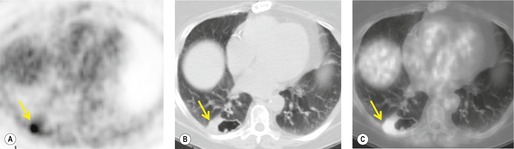
FDG-PET and PET-CT may be useful in evaluating patients with NSCLC and questionable pleural involvement (Fig. 13.39). The examination has proved highly sensitive in small series, 237 but the specificity has yet to be determined, because the number of benign effusions in reported series is small. The ultrasound demonstration of a pleural mass indicates neoplastic involvement, but other signs such as echoes or septations within the fluid or sheetlike pleural thickening are seen in both benign and malignant pleural effusions. 238
Staging lung cancer: a summary
The prime issue for the surgeon is whether the tumor can be completely removed at thoracotomy whereas the radiotherapist considering radical radiation therapy needs to know that the tumor volume will be encompassed within a suitable radiation field. Disease quantification and therapeutic response are important for radiotherapists and chemotherapists treating patients deemed unsuitable for surgery.
Staging the intrathoracic extent of NSCLC is a multidisciplinary process employing imaging, bronchoscopy, and biopsy (see Box 13.7). Thoracoscopy can be performed on patients with suspected pleural involvement. Thoracoscopy may also play a part in preventing fruitless surgery in patients with no CT evidence of nodal enlargement by enabling inspection of pleural surfaces and access to lower mediastinal nodes. 239 Chest radiography and CT, along with PET-CT, are currently the routine imaging procedures for assessing intrathoracic spread and determining resectability, with MRI and ultrasound reserved for specific indications. Based on the studies published so far, MRI has not demonstrated enough advantages to replace chest CT as a routine staging procedure, though it can, in highly selected patients, be useful as a problem-solving technique.
Box 13.7
• CT sensitivity and specificity for mediastinal nodal disease is about 65%, therefore targeted biopsy of enlarged nodes is required
• CT predicts resectable tumors, but is unreliable for identifying inoperable mediastinal invasion
• MRI is comparable to CT as a routine test, but can be useful for solving specific problems
• PET is an accurate technique for diagnosing nodal disease but has no proven value for determining the extent of the primary tumor
• Ultrasonography has a limited role
• Patients should not be denied surgery based on indeterminate imaging findings
The poor specificity of chest CT for determining nodal involvement must be appreciated. Nodal enlargement, although it may be due to metastatic carcinoma, may also be due to coincidental benign disease or to reactive hyperplasia directly connected to the presence of the tumor. Thus, in practice, CT and MRI examinations for staging nodal involvement are used largely to decide whether to perform mediastinoscopy or mediastinotomy and, equally importantly, to demonstrate which nodes should be biopsied. A convenient policy is to consider nodes with a short-axis diameter of greater than 10 mm to be abnormal. Nodes above this diameter should be subjected to some form of biopsy. Clearly there are occasions when the chances of a negative biopsy are so slim that a surgeon may decide that the presence of greatly enlarged nodes is sufficient reason not to proceed with surgical resection, but these cases should be the exception to the general rule that the imaging diagnosis of mediastinal nodal metastases should be corroborated by biopsy before a patient is denied potentially curative surgery. Routine mediastinoscopy provides access only to the paratracheal nodes, proximal tracheobronchial nodes, and superior subcarinal nodes. The other nodal sites require alternative approaches such as mediastinotomy or PET. These other sites include nodal stations with a high propensity to early metastases, such as the aortopulmonary and anterior mediastinal nodes.
Some thoracic surgeons believe it appropriate to proceed to thoracotomy without prior mediastinoscopy or mediastinotomy in patients with normal-sized mediastinal nodes. 186 Others advocate routine mediastinoscopy even in those patients whose CT scans do not show enlarged nodes. 240 A randomized controlled trial of the use of CT in 685 patients with apparently operable lung cancer (with mediastinoscopy for those patients who showed enlarged nodes or thoracotomy without prior mediastinoscopy for those patients who showed no enlarged nodes) versus no CT (all patients having mediastinoscopy) showed that the strategy of using CT to determine which patients should have mediastinoscopy is likely to produce the same number or fewer unnecessary thoracotomies in comparison with doing mediastinoscopy on all patients, and is also likely to be as or less expensive. 241 Another argument against routine preoperative mediastinoscopy of patients with normal-sized nodes is that patients with microscopic metastases discovered only at the time of thoracotomy have an improved survival rate if the primary tumor and the affected mediastinal nodes are resected.
PET has proved significantly more accurate than CT for diagnosing or excluding mediastinal nodal metastases, but has not obviated the need for histologic confirmation of nodal involvement when the PET is positive. Patients with no enlarged nodes on CT and no abnormal uptake of FDG on PET have such a low incidence of nodal involvement that mediastinoscopy is very unlikely indeed to be positive and can be omitted.
For lung cancers that have invaded the mediastinum or chest wall, the important decision is whether the tumor is nevertheless resectable for possible cure, recognizing the poorer prognosis compared with tumors confined to the lung. CT may show definitively that the tumor is too extensive for resective surgery. Alternatively, CT may leave the issue in doubt, and MRI can then be used as a problem-solving technique, but the introduction of multiplanar CT has significantly reduced the number of occasions when MRI is needed. In practice the use of MRI is largely limited to evaluating superior sulcus tumors, to define brachial plexus and subclavian and axillary artery involvement, and for establishing vertebral body involvement. 110
The most important single message is that patients with primary NSCLC should not be denied potentially curative surgery based on indeterminate imaging findings.
Imaging extrathoracic metastases from lung cancer
A reasonable approach for patients with no clinical features to suggest extrathoracic metastases is to extend the staging chest CT to image the liver and adrenals at the time of the thoracic staging scan. In most centers no further routine imaging is undertaken, and there is good evidence to support this approach. 110 In centers where FDG-PET imaging is readily available, it should be used.
The wider availability of FDG-PET imaging currently changes the approach to diagnosing asymptomatic extrathoracic metastases, because it is a single test which can encompass all the organs in the body. The results so far suggest higher accuracy than CT, MRI, ultrasound or most other radionuclide procedures for all body sites, apart from the brain. In particular, PET has been shown to be highly sensitive.205. and 242.
Whole-body PET alters management in a significant proportion of patients, the proportion ranging from 24% to 65%. 213 A randomized prospective trial in 188 patients with suspected operable NSCLC showed that the addition of PET to the conventional workup prevented unnecessary thoracotomy in 20%. 213 Nine studies comprising 837 patients examining the utility of FDG-PET were reviewed by Shon et al. 204 FDG-PET detected 94% of all metastases, considerably higher than a combination of the other standard tests. PET was the only technique to correctly exclude metastatic disease in 53 patients in whom other imaging had suggested metastases. Put another way, 243 FDG-PET has been shown to detect occult extrathoracic metastases in 11–14% of patients selected for curative resection by conventional methods, 201 with two studies244. and 245. having no false-positive results. Higher detection rates are seen with more advanced tumors. 246
FDG-PET is relatively poor at detecting cerebral metastases, due to the high metabolic activity of normal brain. 204 PET is superior to technetium-99m radionuclide bone scintigraphy for the detection of bone metastases. It had a 92% sensitivity and 99% specificity compared with a 50% sensitivity and 92% specificity of bone scans in one series. 201 Apart from issues of availability and cost, the level of accuracy of PET could potentially eliminate the need for radionuclide bone scans. The results from whole-body imaging using PET suggest it is much more sensitive than CT and is also more specific in the detection of hepatic and adrenal metastases. 204
Moving patient platforms with integrated surface coils have made whole-body MRI possible within a single examination. 247 Whole-body MRI allows an overall assessment for metastases in the entire body. In one recent study of 203 patients with lung cancer, MRI had an improved sensitivity and specificity for the detection of metastases as compared with PET-CT. 248
Liver metastases
The presence of clinical and laboratory features suggestive of liver disease has only a 25% positive predictive value for metastases. 249
The advent of multidetector CT provides rapid scanning with several technical advantages, including the optimal use of intravenous contrast medium, the limitation of respiratory misregistration, and the reduction of partial volume averaging by using overlapping image reconstruction or retrospective thin sections. The use of narrow reconstruction intervals can result in an increased detection rate of small lesions. An accepted figure for the accuracy of CT in detecting metastatic disease, extrapolated from studies of colorectal tumors,250. and 251. is approximately 85%, and given the appropriate circumstances biopsy confirmation is not usually required. A major diagnostic problem is distinguishing between small metastases and incidental benign lesions, such as simple cysts/hemangiomas.
Ultrasound is also a suitable method to search for liver metastases, although it is less sensitive than CT. Most deposits are hypoechoic, although hyperechoic and targetlike appearances occur. Recent technical advances mean that body MRI is now superior to CT for imaging liver metastases. Greater tissue contrast results in increased sensitivity for the detection of metastases. However, the cost and speed limitations of MRI mean that it is currently reserved to solve specific problems. 252
Adrenal metastases
The adrenal gland is a common site for metastasis.249. and 253. However, incidental nonfunctioning adrenocortical adenomas are relatively common in patients undergoing CT, with a frequency of approximately 1%. A small solitary adrenal nodule in a patient with NSCLC is more likely to be an incidental benign adenoma than a metastasis. A study of 546 patients with lung cancer found one or more adrenal masses in 4% of patients. On percutaneous biopsy only 29% of these proved to be malignant. 254 The relative incidence of benign adenoma and metastasis reverses when an adrenal mass is more than 2 cm in size.
Given that there is a substantial chance that an adrenal mass of less than 2 cm will be an incidental adenoma, 253 the diagnosis must be confirmed with a high degree of certainty in order to prevent an otherwise operable patient being denied surgery. Both MRI255 and PET256 provide good, but not perfect, results in differentiating benign from malignant adrenal lesions (Fig. 13.40).
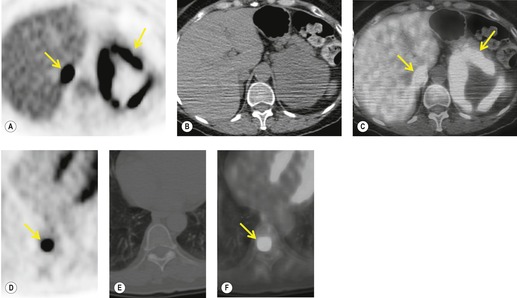 |
| Fig. 13.40 (Courtesy of John Anthony Parker, Boston, MA, USA.) |
Brain metastases
MRI of the brain is more sensitive and may be more specific for metastases than CT.257. and 258. Cerebral metastases occur commonly in lung cancer, particularly from poorly differentiated tumors and adenocarcinoma.249. and 259. MRI with contrast enhancement is the imaging technique of choice. 258 It has particular advantages in showing lesions in the posterior fossa and adjacent to the skull. Given its overall higher sensitivity, MRI is therefore currently preferred over CT when screening patients with lung cancer for brain metastases.260.261. and 262.
The incidence of a truly ‘silent’ brain metastasis is difficult to determine, and may be of the order of 2–4%, 263 though it depends on the thoroughness of clinical examination and method of detection. There is some logic in limiting cerebral imaging to those patients with the more aggressive histologic types of primary tumors. 264
Bone metastases
Bone metastases are a relatively frequent finding at clinical presentation in patients with lung cancer, although the frequencies vary widely between series.249. and 265. Bone scintigraphy is considerably more sensitive than radiography for their detection. 266 However, radionuclide bone scans have a high false-positive rate because of the presence of coincidental benign skeletal disorders, notably old trauma and degenerative disease, and this limits their value. The prevalence of asymptomatic bone deposits detected by radionuclide bone scan is probably of the order of 3–10%. One study of potentially operable nonsmall cell cancers demonstrated bone metastases in 3.4% of patients on radionuclide bone scans. Ichinose et al. 267 studied the bone scans of 196 patients with NSCLC. Metastatic bone disease was identified in just under 10% and subsequently proven on biopsy or follow-up. Of these true-positive scans for metastases, 94% of patients were symptomatic or had abnormal biochemistry. 267 Another study similarly showed that all patients with proven skeletal metastases had at least one clinical or biochemical indicator of bone involvement, 268 emphasizing that bone scintigraphy should in general be performed only in patients with symptoms suggesting bone metastases.
MRI is both sensitive and specific for diagnosing skeletal metastases, and previous limitations have been overcome with the introduction of whole-body MRI. 248 Finally, the assessment for potential bone metastases is an integral part of staging examinations performed with PET-CT (Fig. 13.40).
Bronchioloalveolar carcinoma
The World Health Organization classifies bronchioloalveolar carcinoma, also known as alveolar cell carcinoma or bronchiolar carcinoma, as a subtype of adenocarcinoma. 269 Some published series contain a mixture of pure bronchioloalveolar carcinoma and adenocarcinoma with bronchioloalveolar features, leading to confusion over imaging findings and survival characteristics. 270 Bronchioloalveolar carcinomas are divided into: nonmucinous, mucinous, and mixed mucinous and nonmucinous (indeterminate cell type) subsets. Cigarette smoking does not appear to play a prominent etiologic role, 271 but the prevalence of this tumor within preexisting lung scars is striking. 272
Bronchioloalveolar carcinomas account for 2–5% of all lung cancers, but with smoking on the decline the relative incidence of bronchioloalveolar carcinoma in some series is rising. 23 The tumor occurs equally in both genders, and the average age at onset is between 55 and 65 years. The characteristic pathologic feature is a peripheral neoplasm showing lepidic growth, the malignant cells using the surrounding alveolar walls as a scaffold. These tumors are believed to arise from type II pneumocytes and probably also from bronchiolar epithelium273 or a common stem cell. The cells produce mucus, sometimes in such large amounts that one of the presenting symptoms in the consolidative form of the disease is the expectoration of large quantities of mucoid sputum.
The tumor presents in two clinically different forms: a discrete solitary pulmonary nodule (sometimes more than one nodule is present), and unifocal or multifocal areas of pulmonary consolidation; the form presenting with a solitary pulmonary nodule being the more common.271.274. and 275. The mucinous type is more likely to be seen as airspace consolidation and the nonmucinous type is more likely to be masslike in configuration and associated with secondary scarring and inflammation, but there is no exact correlation between the cell type and the imaging appearance.276. and 277. The scarring may lead to difficulties in determining whether the carcinoma is an invasive adenocarcinoma with a prominent bronchioloalveolar growth pattern or a bronchioloalveolar carcinoma associated with scarring. 55
Pathologists may find it difficult to distinguish histologically between the nonmucinous form of bronchioloalveolar carcinoma and atypical adenomatous hyperplasia, a benign proliferation of bronchioloalveolar cells that is now believed to be a precursor lesion to adenocarcinoma. This difficulty translates to interobserver variability in the diagnosis of these two conditions.
The prognosis for bronchioloalveolar carcinoma when it occurs as a solitary pulmonary nodule is better than that seen with other lung cancer cell types. 278 It is more likely to be stage I and therefore surgically resectable. Also, the tumor is relatively slow growing, 271 so that 5-year survivals are better, regardless of stage. 279 Bronchioloalveolar carcinomas less than 2 cm in diameter, showing a purely lepidic growth pattern without invasion, had no lymph node metastases and 100% 5- and 10-year survival in one series. 55 The prognosis for the larger, more ill-defined lesion, which radiographically resembles pneumonia, and for the disseminated form are both poor.275. and 280.
Because bronchioloalveolar carcinomas arise from the alveoli and the immediately adjacent small airways, they tend to appear as peripheral pulmonary opacities. The most common appearance is a solitary lobulated or spiculated pulmonary nodule of soft tissue density indistinguishable from other types of carcinoma (Fig. 13.41).281.282. and 283. There is a propensity to a subpleural location and the development of a pleuropulmonary tail; the tail is due to desmoplastic reaction in the peripheral septa of the lung.283. and 284.
The nodular form may show a definite air bronchogram, a phenomenon that is best seen with CT,282. and 285. particularly high-resolution (HR) CT.281. and 286. Bubblelike lucencies corresponding to patent small bronchi or air-containing cystic lucencies are a frequent finding.66.281.282.285. and 286. The bubbles, as in the nodular form, correspond pathologically to small patent bronchi or cystic spaces within the tumor. 287 It is a particular feature of the goblet cell subtype of bronchioloalveolar carcinoma. 288 The tumor may be composed entirely of ground-glass density or there may be a halo of ground-glass density, surrounding the central soft tissue density.52.281.285.289. and 290. Frank cavitation, however, is unusual, but an appearance resembling cavitation may be produced by paracicatricial emphysema, fibrosis with honeycombing, and localized bronchiectasis. 291
The radiographic presentation that distinguishes bronchioloalveolar carcinoma from the other types of lung cancer is when the lesion takes the form of single or multiple areas of consolidation or ill-defined opacities. Bronchorrhea is a recognized clinical manifestation in this form of the disease. A variety of radiographic, CT, and HRCT appearances are seen with this form of the disease:277.283. and 292. ill-defined consolidation, resembling pneumonia; pure ground-glass (Fig. 13.42),285.289.290. and 293. ground-glass surrounding a core of soft tissue density, mixed ground-glass opacity and denser consolidation, 270 homogeneous consolidation of one or more lobes (Fig. 13.43), 280 multifocal patchy consolidation, 294 or multiple ill-defined nodules spread widely through multiple lobes in one or both lungs, which may show a centrilobular distribution on HRCT292. More than one of these patterns is often present simultaneously (Fig. 13.44). 292 Extensive, patchy, ground-glass opacities and septal thickening, resembling alveolar proteinosis, has also been described (Fig. 13.44).295. and 296. Both atelectasis and expansile consolidation (Fig. 13.45) have been reported. 297
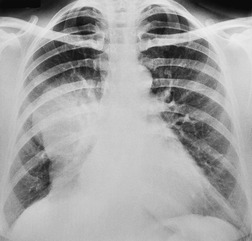 |
| Fig. 13.45 |
Cavitation within consolidation is unusual but has been recorded, as have multiple thin-walled cystic lesions at CT within or coexistent with the consolidative form of bronchioloalveolar carcinoma.292. and 298. In one reported case the wall of a preexisting lung cavity became thickened, presumably by tumor growing around the wall of the cavity. 297
Air bronchograms may be an obvious feature (Fig. 13.42 and Fig. 13.44); they are particularly well seen on CT. 286 The bronchi may show uniform narrowing and stretching. 299 CT may show a ‘bubblelike pattern’ (sometimes referred to as ‘pseudocavitation’) (Fig. 13.42 and Fig. 13.44) in the consolidative form as well as in the nodular form. 66 Thickened septal lines caused by lymphatic permeation may also be visible, as may branching tubular densities of mucoid impaction. 297 Mucus within the tumor may be visible as ground-glass opacity or consolidation at CT. 286
Differentiating between the consolidative forms of bronchioloalveolar carcinoma and various non-neoplastic conditions such as pulmonary infection, organizing pneumonia, aspiration, or pulmonary edema depends on knowing the clinical findings and appreciating the lack of response to treatment. The presence of coexistent nodules along with the consolidation and a peripheral distribution proved to be a statistically significant predictor of bronchioloalveolar carcinoma rather than pneumonia. 300 In another study, stretching and squeezing of air bronchograms traversing the consolidated areas, and bulging of fissures, were significantly more frequent in bronchioloalveolar carcinoma than in pneumonia. 301 The appearances have also been compared with tuberculous pneumonia, and it was shown that while both entities had a similar combination of signs, the ground-glass opacity in bronchioloalveolar carcinoma tended to be remote from the consolidation, whereas in tuberculosis it was adjacent. 292 Also, the pulmonary abnormalities showed a lower lobe predominance in bronchioloalveolar carcinoma, but an upper lung predominance in tuberculosis. There is increased interest in bronchioloalveolar carcinoma manifesting as ground-glass nodules.56.58. and 59. One study investigated the predictive potential for malignancy in these lesions and found that lesion size and morphological characteristics enhance radiologists’ performance in determining malignancy of pure ground-glass nodules. 59 Another study compared the clinical and imaging characteristics of solitary and multiple ground-glass nodules. 58 The authors found that clinical, pathological, and CT features of persistent multiple ground-glass opacity nodules differed from those of solitary ground-glass opacity nodules. Nevertheless, the authors concluded that the two nodule types can probably be followed up and managed in a similar manner because their prognoses are similar. 58
An interesting sign is the CT angiogram sign. It refers to clearly visible vessels coursing through the tumor on contrast-enhanced images, presumably because of the contrast against the background of abundant low-density mucus within the neoplasm. This sign was described before the advent of faster injection rates for contrast agent consequent on spiral CT; it was present in a high proportion of patients with lobar consolidation caused by bronchioloalveolar cell carcinoma, but was unusual in consolidation from other causes. 302 The sign is now regarded as nonspecific, since it is seen in many conditions, notably pneumonia and lymphoma.303. and 304. Pleural effusions are seen in up to one-third of patients, and hilar and mediastinal lymphadenopathy is seen in close to one-fifth.
CT is advocated as a routine before surgery in all patients with the consolidative form of disease to make sure that the tumor is confined to one lobe, since CT frequently shows further pulmonary foci that are not appreciated on plain chest radiographs. 305 MRI provides little extra information compared with CT. Ground-glass opacities, an important CT feature, are not visible on MRI, but very heavily T2-weighted sequences can show the presence of mucin as high signal. 306 The nodular forms of the tumor show enhancement following gadolinium enhancement. 306
Bronchioloalveolar carcinomas may grow slowly, with doubling times far greater than the 18 months usually quoted as the upper limit for bronchial carcinoma. 284 Similarly, reflecting the slow growth and hence relatively low metabolic activity, FDG-PET shows less uptake in bronchioloalveolar carcinoma than other cell types and may be negative.295. and 307.
Recurrence of treated lung cancer
There are only one or two reports in the literature on imaging local recurrence of the primary tumor following treatment and no generally accepted imaging protocols for follow-up of patients who have been or are undergoing treatment for lung cancer. It needs to be borne in mind that recurrent tumor is rarely amenable to cure; a retrospective survey of the experience at the M D Anderson Hospital in the USA showed that only 3% of patients with recurrent tumor were even offered potentially curative treatment. 308 Also, the survival benefit of any form of routine imaging follow-up has yet to be demonstrated. 309 Routine CT or MRI is not recommended, because they are both insensitive and nonspecific for detecting posttreatment recurrence; the only recommended follow-up technique for asymptomatic patients is chest radiography310 and possibly radionuclide imaging, notably FDG-PET.
The signs of local recurrence on chest radiography, CT and MRI are, in general, similar to those used to diagnose the original tumor. CT has been shown to be significantly more sensitive than chest radiography. 311 The general principle is recognizing or being able to exclude an abnormal mass, particularly a growing mass, or enlarging mediastinal lymph nodes. This is relatively easy to do following segmental resection, or lobectomy, because these two surgical procedures do not, in general, lead to confusing degrees of scar formation. Following pneumonectomy or radiation therapy, however, posttreatment fibrosis can make it very difficult to distinguish benign scar tissue from recurrent tumor. The consolidation and fibrosis of radiation pneumonitis may be impossible to distinguish from neoplastic tissue. Libshitz et al. 312 made the interesting observation that opacification of previously air-filled dilated bronchi in areas of postradiation fibrosis is a reliable sign of locally recurrent lung cancer.
Radionuclide imaging, notably FDG-PET, would appear to be the most accurate technique for diagnosing recurrent tumor.313.314.315.316. and 317. Since all forms of radionuclide imaging depend on the altered function of neoplastic cells rather than disordered anatomy, the distorted scarred background is not an impediment to diagnosis (Fig. 13.46). Shon et al. 204 in a review of the literature stated that FDG-PET has been shown to be highly sensitive in the detection of recurrent lung cancer with sensitivities ranging from 97% to 100%, but the false-positive rate, while not as poor as with CT or MRI, could be as high as 40%. The advice is to wait at least 3 months, and preferably 6 months after completion of radiation therapy before performing FDG-PET imaging in order to avoid false-positive results due to radiation fibrosis and glycolysis in macrophages in successfully treated tumors.204. and 317. Sequential scans can reduce the false-positive observations because uptake in non-neoplastic tissue decreases with time.
Atypical adenomatous hyperplasia
Atypical adenomatous hyperplasia (AAH) is a relatively recently described pulmonary neoplasm. On a genetic basis, it is considered by some, on the basis of genetic similarities, as a preinvasive form of pulmonary adenocarcinoma. 318 Other authors suggest that AAH and the associated cancer are genetically independent and that AAH foci could represent an early spread of cells from the main tumor, rather than a precursor lesion. 319 The lesion is defined by the WHO269 as ‘a focal lesion, often 5 mm or less in diameter, in which the involved alveoli and respiratory bronchioles are lined by monotonous, slightly atypical, cuboidal to low columnar epithelial cells with dense nuclear chromatin, inconspicuous nucleoli and scant cytoplasm’. Atypical adenomatous hyperplasia was originally reported as an incidental discovery in autopsy patients without lung cancer, and in up to 10% of lobes removed from patients who had had surgery for bronchial carcinoma, notably adenocarcinomas.320.321. and 322.
The precise nature of the lesion is debated. The debate centers on whether the condition is a highly differentiated adenocarcinoma and part of a continuous spectrum with nonmucinous bronchioloalveolar carcinoma, or whether the lesion is a potentially malignant benign neoplasm, in other words a precursor to bronchioloalveolar carcinoma/adenocarcinoma.323.324.325.326.327.328.329.330.331.332. and 333. The lesions are mostly too small and of insufficient density to be seen on chest radiography, but they can be seen on CT as a round area of ground-glass density, usually less than 10 mm, but sometimes as large as 32 mm in diameter.323.334.335. and 336. There may be a solid portion of soft tissue density in the center of the ground-glass density in a substantial number of cases.332. and 336. Another important feature is the lack of change on follow-up CT examinations. 336
Population screening for lung cancer
The underlying concept of an early lung cancer detection program is that more cures are achieved when stage I tumors are found in asymptomatic individuals, since the surgical results and 5-year survival figures are much better, compared with patients who first present with symptoms and those who have more advanced tumors. Considerably more than half of all patients currently present with stage III disease or higher and it is well accepted that the overall average 5-year survival is somewhere between 13% and 15%,337. and 338. whereas 5-year survival rates for stage IA NSCLC are 67% or better.99. and 163.
It is generally held that the measure of success of a lung cancer screening program should be reduced mortality from lung cancer rather than improved 5-year survival, which suffers from lead-time bias (see below). It has however been suggested that other outcome measures are more appropriate, notably fatality rate and all-cause mortality (see Table 13.6 for definitions). The major problem with using fatality rates is inherent overdiagnosis bias: if lesions that grow too slowly to kill the patient during their natural life expectancy are included in the denominator, the proportionate number of deaths is reduced and the test appears more beneficial than if only truly life-threatening cancers are included. All-cause mortality is a good measure, but is only a true guide in meticulously randomized trials.
| Survival | Number diagnosed with cancer alive/total number diagnosed with cancer (%) |
| Mortality | Number of cancer deaths/total number screened or in control group (deaths per 1000 per year) |
| Fatality | Number of cancer deaths/number of cancers detected (%) |
| All-cause mortality | Number of deaths/number of patients screened (deaths per 1000 per year) |
Biases in cancer screening programs
The presumption that earlier diagnosis necessarily equates to a decrease in mortality from lung cancer is simplistic because there are so many variables to take into account.339.340.341.342.343. and 344.
Screening programs which are compared simply with historical or nonrandom controls suffer from three fundamental biases: lead-time bias, length bias, and overdiagnosis bias. Such programs have the following characteristics: earlier stage at diagnosis, improved resectability rates and improved survival, but no change in the number of late stage tumors and, most importantly, no reduction in mortality from the tumor. The inherent biases can be minimized by comparing disease-specific deaths (deaths due to lung cancer) in a randomized controlled trial of a screened versus a nonscreened population with sufficiently long follow-up to compensate for lead-time bias. A fourth potential bias operates even in randomized trials, namely selection bias, where conclusions may be based on series of patients in whom the randomization procedures do not result in truly similar groups which may not be representative of the population at large.
The term ‘lead-time bias’ refers to the extra life expectancy that occurs simply from diagnosing a tumor earlier, regardless of whether or not treatment is effective. In other words, moving the time of diagnosis of a lung cancer forward inevitably improves 5-year survivals, which are calculated from the time of diagnosis, regardless of the effect on mortality.
The term ‘length bias’ refers to the tendency for tumors with an inherently better prognosis, e.g. slow growing bronchioloalveolar carcinoma, to be discovered by population screening, particularly on the first round (referred to in screening parlance as the ‘prevalence’ round). An indication that length bias can be a highly significant factor is the observation that the volume-doubling times of the lung cancers found in one of the two large Japanese CT screening programs345 was a mean of 15 months (range 1–40 months); by way of comparison the vast majority of lung cancers found by means other than CT screening double their volume with median times of between 4 and 7 months.346.347.348.349.350. and 351.
The term ‘overdiagnosis bias’ refers to overdiagnosing a benign lesion as a malignant tumor, or diagnosing a very slow growing malignancy that would not kill the patient during his or her natural life expectancy. 339 Overdiagnosis bias is an extreme form of length bias. Atypical adenomatous hyperplasia, believed to be either a benign process or a preinvasive carcinoma, is found in a small but significant proportion of patients in all CT screening programs.326. and 334. It resembles the nonmucinous form of bronchioloalveolar carcinoma histologically,276. and 330. and histopathologists vary considerably among themselves over whether they designate very small lung lesions as atypical adenomatous hyperplasia, or whether they classify the lesion in question as invasive carcinoma. Another possible pointer to overdiagnosis in CT screening is that in one of the Japanese screening studies the rate of detection of lung cancer was similar among smokers and nonsmokers, 352 whereas mortality from lung cancer is heavily slanted to smokers.
The arguments against overdiagnosis being a significant factor center on: first, the comparatively few lung cancers demonstrated at screening CT of high-risk populations (the highest reported prevalence in population screening is 2.7%353) and on preoperative CTs of patients undergoing lung volume reduction surgery for emphysema where the lung cancer detection rate is between 2% and 5%;354.355.356. and 357. and, second, the low incidence of previously unsuspected indolent cancers at routine autopsy of older individuals. 358 The reply from those who believe that overdiagnosis is a significant factor is that small lung cancers are not looked for carefully enough at routine autopsy and evidence has been provided to support the claim that the true incidence is higher than reported. 359
There are conflicting data regarding survival and fatality from the small tumors likely to be found by population screening with CT. Patz et al., 360 rather than ask ‘does population screening diagnose lung cancer earlier’, investigated whether there was any correlation between survival and precise size in 510 patients with tumors under 3 cm in diameter without nodal or distant metastases (T1N0M0 or stage IA tumors). Rather unexpectedly, they found no correlation; in other words, the patients with lung cancers less than 1 cm in diameter did not show better survival than patients whose tumors were 2–3 cm in diameter. Their point was that survival following surgical resection from lung cancer is complex and multifactorial, depending not only on tumor size and stage, but also on tumor biology, the ability of body defense systems to cope with micrometastases, and a variety of other host factors. 361 Koike et al., 362 on the other hand, found that 5-year survival for patients with tumors less than 2 cm in diameter was significantly better than for patients with tumors between 2 cm and 3 cm in diameter, a difference that could be explained by lead-time bias alone, particularly as there was no significant difference in the rate of mediastinal nodal involvement between the two groups. Henschke et al. 363 approached the same basic question in yet another way by analyzing the 8-year fatality rate of unresected T1 lung cancers of varying size in the National Institutes of Health Surveillance, Epidemiology, and End Results (SEER) registry and found that almost all lung cancers, even those less than 15 mm in diameter, are fatal if not treated.
Population screening for lung cancer using chest radiography
Two large randomized controlled trials of population screening using chest radiography have been undertaken, the NCI (National Cancer Institute) Mayo Lung Project364.365. and 366. and the Czechoslovakian study367 as well as a number of trials of less rigorous design.368.369.370.371.372.373. and 374. The results have been broadly similar.
The multicenter survey conducted by the NCI between 1971 and 1983 has been extensively analyzed and is the only one that will be discussed in any detail. From a population of approximately 10 000 high-risk patients at each of three centers (men over 45 years of age who were chronic excessive cigarette smokers), 0.73% had lung cancer diagnosed at their initial screening.364.365.375.376.377.378. and 379. After excluding nearly 1000 individuals who were ruled ineligible because of serious medical problems, the Mayo Clinic portion of the study randomly assigned half of those who did not have cancer at the initial screening to surveillance by chest radiography and sputum cytology every 4 months, the remaining half, who served as control subjects, were recommended to have annual chest radiographs and sputum cytology.365. and 380. The rate of cancer diagnosis in the screened group was 5.5 per 1000 per year, compared with 4.3 per 1000 per year in the control group. The resectability rate was higher in the study group (46%) than in the control group (32%) and the median survival was at least three times better, but after a median of 20 years the lung cancer deaths were virtually identical in the two groups: 4.4 per 1000 in the screened arm compared with 3.9 per 1000 in the control group. 366 The apparent disparity between the greatly improved survival and the lack of improvement in mortality from lung cancer is believed to be due to overdiagnosis in the screened population.366. and 381. Another piece of evidence pointing to biases in the study is that 70% of the cancers that had been overlooked on previous chest radiographs were still stage I when finally diagnosed. 28 Huhti and colleagues340 also noted that, when the diagnosis was previously missed, the survival rates were better.
Two of the three centers investigated the benefit of using sputum cytology as an adjunct to chest radiography. Some 15–20% of patients with cancer showed malignant cells in the sputum when the plain chest radiograph was normal. Interestingly, these patients, who all had either centrally situated squamous cell carcinoma or mixed histologic features with squamous cell carcinoma as one element, had a more favorable prognosis than those who had positive radiographic findings.28. and 365. However, neither study showed that the addition of sputum cytology reduced mortality from lung cancer.376. and 382.
The conclusion was that large-scale radiographic and cytologic screening for lung cancer did not result in a mortality benefit. Critics of this conclusion have argued that there might have been a benefit to a subset of patients and that the chest radiography component of the study only had the power to show a reduction in mortality of 50%, rather than a more realistic expectation,81.381.383. and 384. but a subsequent detailed reanalysis of the data has confirmed the initial conclusions of no significant reduction in the death rate: 95% confidence intervals allowed for at most only a tiny improvement in mortality. 366 The initial hope that intensive screening would detect a large proportion of early cases of squamous cell carcinoma, the cell type with the best surgical result, was also not fulfilled. 364
Population screening for lung cancer using low-dose CT
Low-dose CT has been used in several centers as the primary technique for screening an at-risk population for lung cancer.
The points to note from the CT screening programs are:
• The diagnostic yield of lung cancer from CT is far superior to chest radiography.353.385.386.387. and 388. At least twice as many small lung cancers are visible on CT than on chest radiography. The lesions that are invisible on chest radiographs are either below the threshold for noncalcified nodule detection or, when above this threshold, have infiltrating and, therefore, relatively ill-defined margins. 389
• A very high proportion of the cancers diagnosed are stage IA (Fig. 13.47), fulfilling one of the primary objectives of screening, namely to diagnose asymptomatic early stage disease.
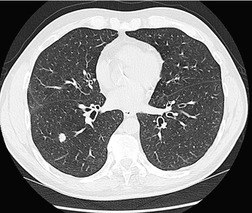 |
| Fig. 13.47 (Courtesy of Dr. Mary Roddie, Medicsight, London, UK.) |
• The cancer diagnosis rate is highly dependent on risk factors inherent in the inclusion criteria, with a much higher incidence of lung cancer when the age of those screened is above 60, and when screening CT is limited to those with a strong smoking history.
• The pick-up rate of clinically insignificant benign nodules is very high (Fig. 13.48). Between 90% and 97% of uncalcified nodules proved to be benign.
• As expected, the pick-up rate of lung cancers drops considerably in the later rounds of screening. The proportion of lung cancers diagnosed in the later rounds of screening is between a half and a quarter of those diagnosed in the initial round.
The high false-positive rate of screening CT is a major disadvantage. 390 In the ELCAP study, 353 233 of 1000 (23.3%) patients were found to have at least one noncalcified nodule. Therefore, follow-up protocols to assess interval growth were developed for nodules less than 10 mm in diameter, with biopsy limited to those nodules whose growth characteristics make lung cancer likely. In the ELCAP study nodules larger than 10 mm were biopsied. Remarkably, using this algorithm, only 28 of the 233 patients with noncalcified nodules required biopsy. Of these nodules, all but one proved to be malignant.
False-positive rates appear to be even higher in areas, such as the American mid-West, in which there is a very high prevalence of histoplasmosis, but they are also high in Europe, 391 where histoplasmosis is rare. The CT screening study from the Mayo Clinic, with patients from a region with a high prevalence of granulomatous infection, showed that 51% of 1520 screened patients revealed one or more benign noncalcified nodules. 392 It should be borne in mind, however, that several technical factors may have played a role: multidetector CT scanners, narrower collimation, and cine viewing rather than film viewing were used; therefore, the sensitivity for the detection of small nodules would have been higher than in the ELCAP and Japanese studies. Careful assessment of nodules avoids thoracotomy for a substantial proportion of benign nodules, but benign nodules are being removed surgically even in the best surgical centers. 391
The false-positive rate is also surprisingly high in the subsequent rounds of screening. The ELCAP study found 30 new nodules on 1184 rescreens, 22 (73%) of which proved to be benign. 393 The Mayo Clinic program390 has reported the findings of two annual incidence screens showing a 12–13% frequency of benign nodules first recognized on the incidence screens. Therefore, the growth rate of new small nodules must be assessed and shown to be consistent with lung carcinoma prior to surgical removal. 393
Appearance of lung cancers detected in screening programs
Cancers detected by CT screening may be: 51
• Homogeneously solid (i.e. wholly of soft tissue density)
• Pure ground-glass density
• Central solid nodule (i.e. soft tissue density) surrounded by glass shadowing, sometimes referred to as a part solid nodule
• Heterogeneous low density and soft tissue density.
The correlations of these CT patterns are:
• The greater the proportion of ground-glass shadowing the more likely it is that the lesion will grow relatively slowly54 and will be a well-differentiated tumor, such as a bronchioloalveolar carcinoma or atypical adenomatous hyperplasia.394.395. and 396. It has been postulated that these CT patterns may correlate with prognosis, with solitary nodules of pure ground-glass opacities being associated with a better prognosis and pure solid nodules being associated with a poorer prognosis. 35
• The less the solid component the less visible the lesion is at chest radiography. 398
Imaging algorithms to determine the nature of a small pulmonary nodule
The basic principles underlying the diagnostic approach to individual nodules detected during a lung cancer CT screening program are similar to those described for a solitary pulmonary nodule first detected on chest radiography. There is, however, a very important difference, namely that follow-up alone is an accepted approach for nodules under 10 mm in diameter. (It is generally regarded as unwise to follow a nodule larger than 1 cm with imaging characteristics compatible with lung cancer because the delay inherent in follow-up would be detrimental to life-expectancy.)
Follow-up alone is an accepted approach for nodules under 1 cm in diameter, largely because of the much higher probability of any particular nodule being an incidental benign lesion. There is, in reality, no practical alternative: such small lesions are not in general suitable for biopsy, PET, or contrast enhancement, and a large majority of patients would be severely disadvantaged by indiscriminate surgical resection. The harm done by the delay inherent in follow-up, which may allow tumors to metastasize in the interval, is still not accurately quantified, 399 but there are indications that the outcome may be affected relatively little. 400 It is hoped that the delay required to check that the growth rate is compatible with lung cancer, thereby avoiding unnecessary surgery, will outweigh the disadvantage of delaying treatment for what should still be a small tumor at the time of surgery. A variety of algorithms for following nodules, which differ in points of detail, have been recommended.29.84.391.392. and 401. An approach advocated by us is:
• Follow-up at 12 months for individuals with one to six noncalcified nodules <5 mm in diameter. The concept behind this recommendation is that, based on expected growth rates, 90% of nonsmall cell lung cancers <5 mm in diameter will still be no bigger than 10 or 11 mm in diameter at an annual follow-up.
• Individuals with more than six nodules, all under 1 cm in diameter, can be assumed to have multiple granulomas (or metastases if there is a known primary which could have metastasized).
• The shape and density of nodules between 5 and 10 mm in diameter should be assessed. If the lesion is clearly linear, Y shaped, or another specifically benign shape, and/or it shows a benign pattern of calcification (see Chapter 3), the lesion can be assumed to be benign.
• If none of these specific benign features is present, and the nodule is of uniform soft tissue density, then follow-up CT after 3 months should be performed. The frequency of follow-up beyond 3 months should be tailored to the estimated range of possible growth rates determined at the first 3-month review. Growth rates are expressed as the time taken for the nodule to double in volume.
• If the nodule is composed of pure ground-glass density, it is reasonable to delay the follow-up CT for 6, or even 12, months, because the chances are strongly in favor of a benign process, notably infection, atypical adenomatous hyperplasia, or a slow growing tumor such as a bronchioloalveolar carcinoma.
• The total follow-up period should be sufficient to establish the growth rate: (1) a nodule that shows no growth over a 2-year period is very unlikely to be malignant, although it is important to emphasize that 2-year stability does not totally rule out lung carcinoma, and in particular does not exclude bronchial carcinoid; and (2) a nodule that shows a volume doubling time compatible with lung cancer, namely between 1 and 18 months, should be assumed to be a carcinoma and treated appropriately.
• For those centers with ready access to PET, it is reasonable to perform PET on nodules above 5 mm in diameter and expedite biopsy for a nodule which shows abnormal uptake of the tracer. It is important to recognize, however, that a negative PET for nodules in the 5–10 mm range is unreliable evidence of benignity.
• A cluster of nodules (i.e. tow or more nodules, none of which is >1 cm distant from an adjacent nodule), all of which are <1 cm in diameter, should be regarded as being due to tuberculosis or histoplasmosis; there is a very low probability of any individual nodule being lung carcinoma.
• Above 1 cm in diameter, the approach is identical to that described for a solitary pulmonary nodule discovered on plain chest radiography.
It is important not to underestimate the difficulty in accurately determining the growth rate of nodules under 1 cm in diameter. A nodule only has to increase its diameter by 26%, to double its volume. For example, a 5 mm nodule which doubles in volume during a 6-month period, will increase by just 1.25 mm, a difference which requires meticulous methods of nodule measurement, preferably using dedicated software. 402 With appropriate software it is possible to achieve accuracies for volume measurement with phantom nodules of within 3%, and it is possible to detect in vivo growth after 30 days, even in cancers growing at average rates.84. and 403. Three-dimensional computerized techniques demonstrate asymmetrical growth better than two-dimensional displays, a feature that can be diagnostically useful. 403 There may be a surprising degree of variation between volume measurements of small nodules performed using the same software on different occasions, and different results may be obtained even on a single occasion depending on the region of interest or the starting point for the computer calculation. Measurements are subject to several errors, notably related to computer algorithm, image acquisition, and observer variation. 404 To gain widespread application, the various computerized methods will need to become more widely accessible and less labor intensive.
Advances in technology, notably multidetector CT scanners, cine viewing, computerized nodule detection systems and three-dimensional reconstruction techniques,75.404.405.406.407.408.409.410.411. and 412. may improve the ability to detect and accurately characterize lung nodules, but all the available systems overdiagnose nodules compared with human observers and are, therefore, currently used adjunctively along with radiologists.
Radiation dose
All lung cancer CT screening programs use a low dose, by which is meant the lowest dose compatible with diagnosing early lung carcinoma. In practice, all use between 40 mAs and 50 mAs and between 120 kVp and 140 kVp. Several studies have also been undertaken to determine the optimum low dose,413.414. and 415. and the use of filters to reduce dose further. 416 These studies confirmed that an mAs in the region of 25–50 mAs provides images on which it is just as easy to diagnose malignant pulmonary nodules as would be the case with much higher exposures (the study populations included both primary and secondary tumors).
Is CT screening for lung cancer beneficial? (Box 13.8)
The central aim of any population screening program is to do more good than harm at an acceptable cost. The potential beneficial effect of a lung cancer CT screening program is that it might extend life by reducing mortality due to the lung cancer. Potential harmful effects include extra radiation from repetitive CT examinations and the consequences of false-positive diagnoses, notably anxiety due to lead-time, anxiety and complications from unnecessary further imaging, biopsy, and even surgery.
Box 13.8
• The central aim of any population screening program is to do more good than harm at an acceptable financial cost
• The major potential beneficial effect of introducing a chest CT screening program for lung cancer would be a reduction in mortality from the tumor. Potential harmful effects include: extra radiation from repetitive CT examinations and the consequences of false-positive diagnoses, notably anxiety due to lead-time, anxiety and complications from unnecessary further imaging, biopsy, and even unnecessary surgery
• CT can detect lung cancers under 1 cm in size at a time when the tumor is still stage IA.
• There is no conclusive evidence that population screening using CT saves lives in patients with lung cancer
• The debate regarding the advisability of introducing chest CT screening for the early diagnosis of lung cancer centers on how important the various biases are in the face of the clearly demonstrated ability of CT to detect stage IA lung cancers
• It will take a randomized controlled trial with many years of follow-up to determine the answer to the following important questions:
– Does early detection using up-to-date CT techniques result in a measurable reduction in the mortality of lung cancer?
– Can thoracotomy be largely limited to those patients with life-threatening malignancies?
– Can mass screening with CT be performed in a cost-effective manner?
There has been considerable debate in the literature regarding the advisability of introducing large-scale CT screening for the early diagnosis of lung cancer.81.342.383.401.417.418.419.420.421.422. and 423. The debate centers on the significance of the various biases in the face of the clearly demonstrated ability of CT to detect lung cancers under a centimeter in size at a time when the tumor is still stage IA. Two biases, in particular, may be pivotal: length bias and overdiagnosis.
Currently, cost-effectiveness studies have to make assumptions regarding mortality reduction. When such assumptions are made, the cost per year of life in the US healthcare system is estimated at somewhere between US$2500 and US$62 000 per year of life saved, depending on the screening round being analyzed, the frequency of screening, and the prevalence of lung cancer in the population being screened.424.425.426.427. and 428.
It will take a randomized controlled trial with many years of follow-up to determine any mortality reduction and until this vital piece of information is known it will not be possible to calculate the cost in any meaningful way. 422 The three vital questions to answer in future large-scale studies are: does early detection using up-to-date CT techniques result in a measurable reduction in the mortality of lung cancer; can thoracotomy be limited largely to those patients with life-threatening malignancies; and can mass screening with CT be performed in a cost-effective manner?
Three studies that may answer these questions are currently under way:
• The National Lung Screening Trial (NLST) completed enrollment of 50 000 at risk (current or former smokers) men and women randomly assigned to receive either chest CT scan or chest radiography for 3 years. The trial is powered to detect a 20% reduction in lung cancer mortality due to differences in screening methods. Follow-up will continue through 2009, and the first data will be released in 2010. 429
• The NELSON trial is a randomized CT-based lung cancer trial being conducted in the Netherlands and Belgium; CT screening is being compared with no screening in 4000 current or former smokers. The study is powered to detect a 25% decrease in lung cancer mortality, as well as the effects of screening on quality of life, smoking cessation, and an estimate of cost-effectiveness. This is currently the only large-scale randomized trial to compare CT screening with no screening. 430
• The DANTE trial, a randomized trial in Italy for male smokers age 60–74 years, is designed to assess lung cancer-specific mortality over 10 years, comparing 5 years of annual screening by single slice spiral CT scan or annual clinical follow-up; the control group received baseline screening with chest radiograph and sputum cytology. Enrollment of 2472 subjects is completed and the trial will continue until 2010. At initial evaluation, lung cancer was found in 2.2% of the CT group (71% stage I) and 0.67% of the controls (50% stage I). Fifteen percent of subjects had an abnormal CT, and 4% underwent an invasive procedure. Benign pulmonary lesions were found in 19% (6 of 32) of thoracotomies. 431
‘Missed’ lung cancers
The problem of ‘missed’ lung cancers on chest radiography has been highlighted by several series. The miss rate was particularly high in the chest radiography lung cancer screening programs, a particular setting involving the opportunity to undertake meticulous review of recent previous films. In the Mayo chest radiography lung cancer screening project, 28 45 of the eventually diagnosed 50 primary peripheral cancers were visible in retrospect but had been overlooked at the first opportunity. The equivalent figure for central lesions was 12 of 16. Similarly, Heelan and co-workers432 found that 65% of cancers in a yearly screening program had been overlooked on the previous film. Many of the overlooked cancers were very difficult to see even in retrospect.
Austin et al. 433 analyzed 27 patients in whom a potentially resectable lung cancer had been missed in routine practice, which was visible in retrospect on previous films. The mean diameter of lesions was 1.6 cm, and almost all had ill-defined edges. As part of the study, six radiologists reviewed the chest radiographs of 22 cases of missed lung cancer and failed to detect a mean of 26% of the lesions. Most were in an upper lobe and in women. In another analysis of 17 cancers visible in retrospect, the failure to diagnose the cancers at the first opportunity was partly due to failure to see the lesion and partly due to misinterpreting the shadow as ‘inflammatory disease’. 434 In another recent series of 40 NSCLCs that were overlooked on chest radiography at a time when the tumors were potentially resectable, the mean diameter of the missed lung cancers was 1.9 cm and again the great majority were peripheral in location and in an upper lobe. 435 Quekel et al. 436 surveyed chest radiographs of patients with NSCLC in a typical community setting and found that the diagnosis was missed in 19% of 259 patients with lung cancer presenting as a pulmonary nodule. The mean diameter of the missed cancers was 16 mm and the median delay in diagnosis was 472 days. 436 The miss rate was strongly correlated with the presence of superimposed normal structures and size of nodule. Lesions that contain a large proportion of ground-glass density are also more frequently overlooked compared with solid nodules. 437 There have also been reports of lung cancers overlooked at initial chest CT:438.439.440. and 441. the mean diameter of overlooked tumors in one series of 14 patients was 1.2 cm and the maximum 2.0 cm. 440 Most cancers missed at CT are endobronchial in location or are situated in the perihilar region and confused with blood vessels.
Deciding whether the failure to diagnose lung cancer on an initial chest radiograph or chest CT constitutes malpractice is very difficult, since ‘misses’ are inevitable even under the best conditions.442. and 443. If failure to diagnose a lung cancer at the first opportunity was to be regarded ipso facto as malpractice, radiologists would be found guilty of malpractice even though their films and interpretation conformed to the same standards as a group of experienced chest radiologists. Many factors contribute to a radiologist’s failure to detect a lung cancer on a chest radiograph,438. and 443. including the size and shape of the nodule, lesion conspicuity, viewing time, 444 and the visual search patterns used by the radiologist. 444 There may also be intentional or unintentional underreporting, driven by the desire to avoid further unnecessary, invasive, or expensive investigation. 27
A variety of techniques to reduce errors of detection have been recommended or investigated,443. and 445. including high-kilovoltage technique, the use of wide-latitude film, feedback systems,446. and 447. and double-reporting. 448 Comparison with previous normal radiographs allows greater confidence in the diagnosis of small nodules and permits recognition of smaller lesions. Clearly the dividing line between negligence and acceptable practice is difficult to define. Since there are no clear-cut criteria, these decisions, as so often in the medicolegal arena, depend on the testimony of expert witnesses and local laws.442. and 449. It is worth noting, however, that the legal case sometimes hinges as much on the technical adequacy of the radiographs and appropriate communication of the findings, as on the issue of film interpretation.
BRONCHIAL CARCINOID
Bronchial carcinoids (see Box 13.9) are neuroendocrine tumors. 450 They constitute less than 5% of all pulmonary tumors. The age range is wide, extending from adolescence451 to old age. They show a spectrum of microscopic appearances and clinical behavior ranging from a slow-growing locally invasive tumor to a malignant metastasizing tumor with a moderately fast growth rate, but even widely metastatic carcinoids may grow very slowly. 452
Box 13.9
• Constitutes less than 5% of all pulmonary tumors
• Age range from adolescence to old age
• Believed to be part of a spectrum that includes small cell carcinoma
• Two well-described forms: typical carcinoid (85–90%) and atypical carcinoid (10–15%)
• Both forms have good prognosis if resected
• Cushing syndrome due to ectopic adrenocorticotropic hormone (ACTH) production by the tumor is more common than carcinoid syndrome. The responsible tumors may be very small
• Most common site of origin is central bronchi
• Calcification is fairly common
• Somatostatin receptor radionuclide scanning (e.g. with octreotide) is highly sensitive
Pulmonary neuroendocrine tumors have been the subject of considerable controversy, resulting in multiple competing and confusing classification schemes. In the 2004 WHO classification the spectrum of neuroendocrine tumors of the lung ranges from hyperplastic neuroendocrine cell lesions (carcinoid tumorlets and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia, DIPNECH) to the high-grade small cell (SCLC) and large cell neuroendocrine tumors. 453 On histology, typical low-grade carcinoid tumors are composed of cytologically bland cells containing regular round to oval nuclei with finely dispersed chromatin and inconspicuous small nucleoli. The cells are usually polygonal in shape and are arranged in distinct organoid, trabecular, or insular growth patterns with a delicate vascular stroma. Peripheral typical carcinoids have a prominent spindle cell growth pattern, and up to 75% have foci of neuroendocrine cell hyperplasia (DIPNECH) and/or tumorlets (carcinoid foci smaller than 5 mm in diameter) in the adjacent lung parenchyma. Coexpression of these preinvasive neuroendocrine cell lesions does not seem to affect prognosis, although the number of series with long-term follow-up is limited. Cytologic atypia is also characteristic but insufficiently diagnostic in the absence of these features. However, at the individual patient level, none of these features enables a reliable prediction of clinical outcome. Atypical carcinoids present more often with hilar or mediastinal nodal metastases (20–60% vs 4–27%), and they have a higher recurrence rate than typical carcinoids. 2
Typical carcinoids without nodal or other metastases have an excellent outcome following surgical resection.454. and 455. Even when spread to hilar or ipsilateral mediastinal lymph nodes has taken place, 5-year survival is 92%.456.457. and 458. Spread is usually first to hilar or mediastinal nodes, and blood-borne metastases at presentation are unusual. The great majority arise centrally in the main, lobar, or segmental bronchi and may cause cough and occasionally wheezing. Repetitive bouts of pneumonia are common, and bronchiectasis and lung abscess may occur beyond the obstruction; hemoptysis and weight loss are also common.455.459.460. and 461. Centrally located bronchial carcinoids462 may be predominantly intraluminal, assuming a polypoid configuration, may grow within the lumen of the bronchus in a ‘toothpaste-like’ fashion, or may be predominantly extraluminal, in which case they are known as ‘iceberg’ lesions. The mucosal surface is usually smooth; only rarely is it ulcerated to any degree. Atypical carcinoids usually arise peripherally in the lung, and both hemoptysis and pneumonitis are rare. They have a worse outcome than typical carcinoids. 463
Carcinoid syndrome is rare with bronchial carcinoids, unless liver metastases are present. 460 Bronchial carcinoid tumors may secrete ACTH in sufficient quantities to cause Cushing syndrome (ectopic ACTH syndrome), clinically indistinguishable from pituitary-dependent Cushing disease. 464 ACTH-secreting tumors may be more aggressive than the usual typical carcinoid. 465 They may be tiny (Fig. 13.49) but are more often small (Fig. 13.50), and may require meticulous searching with chest CT or radionuclide imaging to reveal their presence. 464 Some are so small that they cannot be found until they grow large enough to be visualized with imaging examinations. It is possible to perform needle aspiration of a suspicious pulmonary nodule in a patient with Cushing disease to analyze the sample for ACTH, 466 but analysis of bronchial lavage fluid is unhelpful.
The chest radiograph is abnormal in most cases of bronchial carcinoid, 467 but in approximately 10% of cases the chest radiograph is normal. The majority arise in the larger bronchi and cause partial or complete bronchial obstruction with consequent atelectasis. Collateral air drift may keep segments aerated when the lesion is in a segmental bronchus; even whole lobes can be kept fully aerated despite complete occlusion of a lobar bronchus. The consequent hypoxia of the affected lung may occasionally cause recognizable local vasoconstriction on radiographs and perfusion scintigraphy. 468 In about 25% of patients with a central lesion the tumor is visible on chest radiographs as a hilar mass (Fig. 13.51).
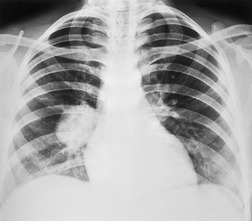 |
| Fig. 13.51 (Courtesy of Dr. Peter Hacking, Newcastle, UK.) |
Small tumors in lobar, segmental, or subsegmental bronchi may cause mucous distension of the bronchi beyond the obstruction (Figs 13.49 and Fig. 13.52, Fig. 13.53 and Fig. 13.54), the surrounding lung remaining aerated by collateral air drift. The resulting bronchocele (mucoid impaction) may then be the dominant feature.
Approximately 10–20% of bronchial carcinoids present as a solitary pulmonary nodule (see Fig. 13.50). The nodule is well-defined, round, oval, lobulated, or notched, usually with a smooth edge, 467 although spiculation is reported. 469 Calcification or ossification is occasionally recognizable on chest radiography. 470 Two large series describing the chest radiographic appearances have been reported, both from the Mayo Clinic.471. and 472. In the earlier series of 23 cases, the average diameter of the nodule was 4 cm; however, the later report on 20 similarly located tumors showed none with a diameter greater than 4 cm. Multiple lesions are a frequent pathological finding, 273 but usually they are tiny and are not recognized on chest radiographs.
CT provides good anatomic localization of both the intraluminal and extraluminal components of tumors in the major bronchi and shows all the imaging features to advantage.473.474. and 475. CT is excellent for showing the details of a central mass, which may be purely intraluminal, a mixture of intra- and extraluminal, or almost entirely extraluminal (Figs 13.52, 13.53 and 13.56). The tumor may narrow, deform, or obstruct the airway. On occasion the bronchial lumen can be seen to widen as it approaches the tumor, a point of distinction from lung cancer (Figs 13.53 and 13.56). Even peripheral carcinoids can be shown to lie adjacent to recognizable small airways.
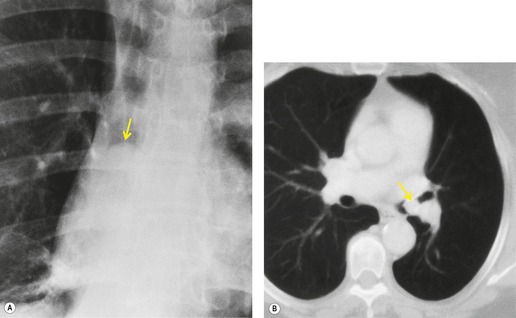 |
| Fig. 13.56 (Courtesy of Dr. Martin Wastie, Nottingham, UK.) |
The incidence of calcification detectable by CT appears to be considerable: four of 12 cases in one series476 and eight of 31 cases in another. 477 The incidence of calcification is significantly greater in centrally located tumors and more frequent in the larger tumors. A variety of patterns of calcification are seen,473. and 477. including multiple nodular and curvilinear configurations (Figs 13.55 and 13.57). Sometimes the calcification takes the form of recognizable ossification and is so extensive that it occupies the whole of the tumor mass.477. and 478. Contrast enhancement, which is sometimes marked, is seen in some cases (Fig. 13.57).479. and 480. Pronounced and rapid contrast enhancement with gadolinium at MRI has also been reported. 481
Carcinoid tumors cannot in general be distinguished from carcinomas on chest radiography and CT unless the lesion is demonstrably heavily calcified, enhances brightly, or shows widening of the approaching bronchus. Small tumors may be difficult to distinguish from the larger blood vessels at CT482 and may, on occasion, be shown to advantage by MRI because on appropriate sequences, particularly T2-weighted or STIR sequences, the contrast between high signal from the tumor compared with the low background signal of normal lung and flowing blood in pulmonary vessels may make the lesions more conspicuous than at CT.
Carcinoids have somatostatin receptors and can therefore be imaged with indium-113 octreotide, a radiolabeled somatostatin analog.483.484. and 485. Somatostatin receptor scintigraphy, while 96% sensitive, is also positive in inflammatory conditions as well as in a variety of other tumors. It shows those carcinoids that are visible on chest radiographs or CT, 483 but has not yet been shown to be of proven value for the diagnosis of ACTH-producing bronchial carcinoid tumors that are not visible on cross-sectional imaging. 486 Other radionuclides that have been shown to accumulate in sufficient quantities to demonstrate a bronchial carcinoid include iodine-123 N-isopropyl-p-iodoamphetamine487 and PET agents such as FDG, 488 and 5-hydroxytrytophan (5-HTP) 489 labeled with 11C and 18F-labeled analogs of octreotide. 490 Currently, however, In-113 octreotide remains the preferred radionuclide in widespread clinical practice for imaging carcinoid tumors.
HAMARTOMAS
Hamartomas are defined pathologically as tumorlike malformations composed of an abnormal mixture of the normal constituents of the organ in which they are found. Most pulmonary hamartomas contain masses of cartilage with clefts lined by bronchial epithelium and fibromyxoid stroma; they may also contain fat or cystic collections of fluid.491.492.493. and 494. Although the precise nature of pulmonary hamartomas is debatable, they are usually regarded as benign neoplasms. 492 They grow slowly and are usually solitary, although there are a few case reports of multiple pulmonary hamartomas.494. and 495. Malignant transformation is either nonexistent or extremely rare,492. and 496. but there are two series showing a higher than expected incidence of lung carcinoma in patients with pulmonary hamartoma.497. and 498. Also, malignant sarcomas arising in the wall of so-called cystic mesenchymal hamartomas have been reported in children, but cystic mesenchymal hamartomas are histologically quite different from the usual hamartoma seen in adults.499. and 500.
The peak age at presentation of pulmonary hamartomas is in the seventh decade of life. 492 They are rarely seen in children. The great majority are situated peripherally, with a few arising in central bronchi.492. and 501. In the largest series published to date, only four of 215 patients with hamartomas had symptoms attributable to the hamartoma, the symptoms being cough, hemoptysis, and recurrent pneumonia. 492
A triad of pulmonary chondroma (often multiple), gastric epithelioid leiomyosarcoma (leiomyoblastoma), and functioning extraadrenal paraganglioma, known as the Carney triad, has been described. 502 The importance of the condition, which is seen mostly in women under 35 years of age, is that, if multiple slow-growing cartilage tumors are found in the lung, the other tumors in the triad should be sought, since the other neoplasms are potentially lethal. Furthermore, patients with a smooth muscle tumor in the wall of the stomach should not automatically be assumed to have pulmonary metastases just because they have multiple pulmonary nodules. An incomplete form of the triad manifesting just the pulmonary chondromata and the gastric smooth muscle tumors is also seen.503. and 504. In addition to the Carney triad, the association of pulmonary hamartomas with other developmental anomalies and benign tumors has been noted. 505
Peripheral hamartomas are seen as a spherical, lobulated, or notched pulmonary nodule with a very well-defined edge surrounded by normal lung (Fig. 13.58). 506 Pulmonary hamartomas can range up to 10 cm in diameter. 507 Large lesions are unusual, however, and most are less than 4 cm. 501 The larger the lesion, the more likely it will calcify. Definite calcification is seen on plain chest radiographs in up to 15% of patients. 501 The calcification may show the typical popcorn configuration of cartilage calcification, in which case the diagnosis is virtually certain (Figs 13.59 and 13.60). The presence of fat density within the mass is a specific diagnostic feature (Figs 13.60 and 13.61). Radiologically detectable air within the tumors is rare, 508 but central lucency caused by fat can be confused with cavitation.
The signs at CT are similar to those at chest radiography, but, because of the better contrast resolution, calcium and particularly fat are more easy to identify (Fig. 13.61). In a series of 47 patients with pulmonary hamartomas it was possible to identify fat (CT numbers in the range −80 Hounsfield Units [HU] to −120 HU) or calcium plus fat within the nodule in 28 patients by use of thin-section CT; this combination appears to be specific for hamartoma, at least in nodules under 2.5 cm in diameter. 509 In 17 of the cases the hamartomas showed neither calcification nor fat, and in the remaining two there was diffuse calcification throughout the lesion. An air bronchogram may be seen within the nodule on rare occasions. 510
Hamartomas show enhancement on CT following intravenous contrast injection,70.71. and 510. but usually the only enhancement is in the surrounding capsule or in septa separating unenhancing components; on occasion, however, there is nonspecific more generalized enhancement. On MRI, 511 hamartomas show intermediate signal on T1-weighted and high signal on T2-weighted images. Septa can be seen separating the nodule into lobules. These septa show marked enhancement following intravenous gadolinium.
TRACHEAL NEOPLASMS
Benign tumors of the trachea are rare. They are most frequent in children, in whom squamous papillomas are the most common. 514 These papillomas are usually part of laryngeal papillomatosis. The next most common benign tracheal tumors of childhood are hemangiomas. They are often associated with cutaneous hemangiomas and are frequently in the subglottic region. Stridor may develop in the first year of life. On imaging examinations tracheal hemangiomas are seen as eccentrically situated nodular masses.
A variety of connective tissue, neural, and other benign neoplasms are occasionally encountered in the trachea, 515 including: lipoma, fibroma, leiomyoma, 516 hemangioendothelioma, cartilage tumors,517. and 518. granular cell myoblastoma (also known as granular cell tumor), 519 laryngeal papillomatosis (Fig. 13.62), and neurilemmoma, neurofibroma, and paraganglioma.520.521. and 522. All these lesions produce a nonspecific focal mass in the wall of the trachea. 514
The most common malignant tumor of the trachea is invasion from an adjacent neoplasm, notably lung cancer. Primary malignant tumors of the trachea are rare and are virtually confined to adults. 515 The most frequent are adenoid cystic carcinoma and squamous cell carcinoma. Mucoepidermoid tumor is the next most common carcinoma: it is more frequent in the major bronchi than in the trachea. 523 Adenoid cystic carcinomas of the trachea present at an earlier age than squamous cell carcinoma, usually between 30 and 50 years. Squamous cell carcinoma is strongly associated with smoking. These three tumors make up over 90% of primary tracheal tumors. The remaining 10% consist of a wide variety of neoplasms, including sarcomas, lymphoma, adenocarcinoma, adenosquamous carcinoma, carcinoid, chondrosarcoma, plasmacytoma, small cell carcinoma, and metastases.516.520.524.525. and 526.
Primary malignant tracheal tumors grow slowly, tend to involve the posterior wall of the lower two-thirds of the trachea, and infiltrate submucosally for long distances.16. and 520. Therefore, stridor and wheezing are common early symptoms. Tracheoesophageal fistula may be the initial feature. By the time of clinical presentation these tumors have often invaded the mediastinum and adjacent esophagus; they are, however, frequently amenable to surgical resection.527. and 528.
All three tumors are seen on imaging studies523. and 529. as an intraluminal nodule or stenosis with lobular or irregular contours (Figs 13.63 and 13.64). The tumors grow within or through the tracheal wall to produce a paratracheal mass and/or a stenosing mass of variable length (Figs 13.63 and 13.64). Low-grade mucoepidermoid carcinomas usually appear as polypoid lesions, a feature most frequently seen with adenoid cystic carcinoma. 529 As with bronchial carcinoid, there may be a relatively large mediastinal mass with a much smaller intratracheal component. Like bronchial carcinoids, mucoepidermoid tumors may travel along bronchi and so adapt their shape to the airways. Like bronchial carcinoid and various benign tumors, adenoid cystic carcinomas may calcify.
The full extent of submucosal spread tends to be underestimated with CT. 530 Although CT shows the extraluminal component of tumors of the trachea, it is poor at indicating whether or not mediastinal structures, such as the esophagus and aorta, are invaded. Obliteration of the fat planes between the tumor and these structures is sometimes caused by invasion, but at other times no invasion is found when surgery is finally performed. 531
RARE MALIGNANT PULMONARY NEOPLASMS
Most sarcomas of the lung are metastases from extrathoracic primary tumors. Primary pulmonary sarcomas are usually fibrosarcomas, leiomyosarcomas, 532 or sarcomas of the pulmonary artery. 533 In a large review from the Armed Forces Institute of Pathology, 534 endobronchial fibrosarcomas and leiomyosarcomas exhibited, for the most part, relatively benign behavior compared with tumors arising within the lung parenchyma, which showed great variability in their degree of malignancy and a significant incidence of highly aggressive behavior. Chondrosarcomas, fibroleiomyosarcomas, malignant fibrous histiocytoma,535.536.537. and 538. osteosarcoma,539. and 540. rhabdomyosarcomas,541. and 542. liposarcomas, which may show fat density on CT, 543 myxosarcomas, neurofibrosarcomas, and angiosarcomas544 may also arise primarily in the lung.
All these sarcomas, except angiosarcomas, appear on imaging examinations as a solitary pulmonary nodule or as an endobronchial mass, usually causing atelectasis, indistinguishable from lung cancer (Fig. 13.65). Angiosarcomas extend or arise intravascularly,538.545.546. and 547. a feature that is well demonstrated on MRI and contrast-enhanced CT, and the tumor itself enhances brightly. A case of pulmonary leiomyosarcoma presenting as a pseudoaneurysm of a peripheral pulmonary artery has been reported. 548
Hemangiopericytomas, which may be benign or malignant, occasionally arise primarily in the lung. The larger the lesion, the more likely it is to be malignant. These tumors are most common in the fourth and fifth decades with no sex predominance. They are usually asymptomatic but cough, hemoptysis, hypertrophic osteoarthropathy, and chest pain are the more common symptoms in larger lesions and there may be associated hypoglycemia or coagulopathy. 549 On imaging examinations, the tumors appear as one or more well-defined pulmonary masses of any size. Speckled, eccentric calcification is seen on occasions. MRI shows a heterogeneous signal pattern corresponding to necrosis and hemorrhage with no specific features.549.550. and 551.
Carcinosarcomas are composed of mixed malignant epithelial and connective tissue components. They are usually first found in middle-aged to elderly patients. Carcinosarcomas may arise centrally or peripherally and are indistinguishable on imaging from other endobronchial or pulmonary tumors. 552
Pulmonary blastomas are malignant tumors which histologically show embryonic bronchial structures in a background of abundant immature sarcoma.273. and 553. Pulmonary blastomas were originally thought to arise from a multipotential mesodermal cell, but like carcinosarcomas they are now believed to arise from two germ cell layers. They are therefore classified as a form of carcinosarcoma. The peak age of clinical presentation is somewhat younger than carcinoma, a small proportion being found in children.551. and 554. Pulmonary blastomas generally arise peripherally in the lung (Fig. 13.66) as a solitary well-defined mass or occasionally as multiple pulmonary masses. 555 Blastomas in adults are often of moderate size but range from 2.5 cm to 26 cm in diameter. Cavitation has been noted on chest radiographs. 556 CT and ultrasound have shown a cystic structure within the mass itself. 557 Calcification has been reported on CT. 554 Pleural effusion may accompany the mass.
Pleuropulmonary blastoma is classified separately.558. and 559. It is an aggressive neoplasm of early childhood, characterized histologically by primitive mixed blastomatous and sarcomatous elements. 560 The tumors may arise in preexisting congenital lung lesions, including cystic adenomatoid malformation, extralobar sequestration, bronchogenic cyst, and pulmonary hamartoma. There are few reports of the imaging features. Chest radiographs and CT show an appearance which resembles pneumonia, but more usually lung cysts, which may be air-filled; in some cases there is an accompanying pneumothorax which may recur.560. and 561. In one report on seven children with ‘pulmonary blastoma’, 558 all the tumors were unilateral and large at initial presentation. The four patients who underwent CT examination showed similar findings: a heterogeneous mass with areas of low attenuation and whorls of high attenuation. In three cases, CT showed a rounded tumor arising within an area of preexisting cystic lung, but they may in themselves be cystlike. The association of pulmonary blastomas and developmental cysts of the lung has also been reported by others. 557 In rare instances the childhood tumor resembles empyema both clinically and on imaging. 551
Primary choriocarcinoma and primary malignant teratoma of the lung are both very rare indeed. The few published cases show a nonspecific pulmonary mass. 562
Plasmacytoma of the lungs or major airways is an exceedingly rare tumor, even in patients with multiple myeloma. With solitary lesions the sheets of plasma cells may be difficult to distinguish histologically from a benign plasma cell granuloma, and some of the cases in the literature may have been wrongly categorized. The most common form of intrathoracic plasmacytoma is inward extension from a rib. Rarely, plasmacytoma presents radiographically as a tracheal, 563 bronchial, or lung mass,564. and 565. or as greatly enlarged intrathoracic lymph nodes. 566 Scattered cases of myeloma involvement of the pulmonary interstitium have also been reported. 567
Askin tumors are malignant small cell tumors of neuroepithelial origin seen in childhood and adolescence, 568 and, rarely, in adults. 569 The great majority arise in the chest wall; 570 a few are almost exclusively in the lung, but even these show pleural involvement. The condition has a poor prognosis, even with combined chemoradiation and surgery, with few long-term survivors, although some are reported. 571 On imaging,572. and 573. there is an intrathoracic soft tissue mass, which may show calcification, and can be huge with either no visible rib destruction or only focal rib lysis. Pleural effusions and hilar adenopathy may accompany the mass.
Epithelioid hemangioendothelioma (intravascular bronchioloalveolar tumor) is a rare cause of multiple small pulmonary nodules. 574 The tumor is believed to be a neoplasm of blood vessels, 575 related to hemangioendothelioma. There is a strong predilection for females, and the age range is 12–61 with a mean in the forties. The patients are often asymptomatic, or have minor symptoms only, and the diagnosis is established following the discovery of multiple perivascular pulmonary nodules, 543 which may mimic interstitial lung disease on chest radiography or CT. 575 The nodules, which may be up to 2 cm in diameter, are well defined or slightly ill defined in outline. They may calcify.576. and 577. Associated pleural effusion may be seen and metastases to hilar nodes are recorded. The course may be indolent with survival of up to 15 years.
Until the advent of acquired immune deficiency syndrome (AIDS), Kaposi sarcoma was regarded primarily as a skin tumor of the lower extremities. The tumor is now being encountered with increased frequency in the bronchial tree and lungs of patients with AIDS and is discussed in the section on AIDS in Chapter 6.
Mucoepidermoid (Fig. 13.67) and adenoid cystic carcinomas may occur in the bronchi but are more common in the trachea.
RARE BENIGN LUNG NEOPLASMS
Alveolar adenomas and papillary adenomas are very rare and present as peripheral solitary pulmonary nodules. 529Salivary gland tumors are usually found in the larger bronchi as solitary, predominantly polypoid or exophytic tumors arising from submucosal seromucous glands and ducts, or occasionally from smaller bronchi within lung parenchyma. 529 They occur in adults and need to be distinguished pathologically from low-grade malignant tumors of the bronchus – particularly mucoepidermoid carcinoma. 578 Radiologically they cause postobstructive atelectasis and pneumonia or present as a solitary pulmonary nodule.
Granular cell myoblastoma is a benign tumor most commonly found in subcutaneous tissues, but may rarely occur in the larger bronchi and even more rarely in the lung parenchyma, 519 trachea, or mediastinum.579.580. and 581. It usually presents in adults as postobstructive atelectasis or pneumonia beyond the lesion itself, occasionally as a small solitary pulmonary nodule, or as a polypoid lesion arising in a bronchus. Multiple lesions are occasionally encountered and hemoptysis may be a presenting feature. 581
Fibroma, chondroma, lipoma, hemangioma, neurofibroma, neurilemmoma, chemodectoma, benign clear cell tumor, fibrous tumor of the pleura, meningioma, and thymoma may arise in the lung parenchyma or in the walls of the bronchi. Those that arise in the lung parenchyma present as a nonspecific solitary pulmonary nodule; those that arise in the larger bronchi are indistinguishable from the more common bronchial carcinoid, except that it may be possible to diagnose fat in a lipoma by CT582 (Fig. 13.68). Endobronchial lipoma has been reported to contain a focus of dense calcification within the tumor, as well as fat density. 583 Most intrathoracic lipomas arise extrapleurally from the chest wall, mediastinum, or diaphragm.584. and 585.
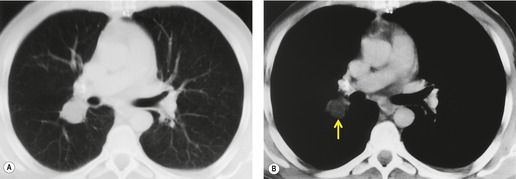 |
| Fig. 13.68 (Courtesy of Dr. Ted A Glass, Fredericksburg, MD, USA.) |
Leiomyoma of the lung may be a solitary lesion, radiographically indistinguishable from the other benign connective tissue neoplasms. Multiple leiomyomas occur as multiple nodules, of uniform soft tissue density in the lung, up to 7 cm in diameter (Fig. 13.69), or very occasionally in the pleura. 586 These tumors are probably very slow-growing metastases from a uterine leiomyoma; there is often a history of previous hysterectomy for uterine fibroids, which may have been up to 20 years previous. 587 Their behavior varies from a benign lesion to a low-grade sarcoma but they are sufficiently indolent that death is commonly from other causes. They are usually asymptomatic and discovered incidentally on chest imaging. 587 The nodules may cavitate showing air–fluid levels and forming very thin-walled, air-filled cavities. A miliary pattern has been described in one case. 588
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree