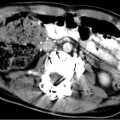
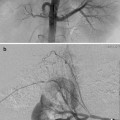
Fig. 24.1
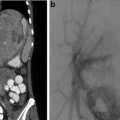
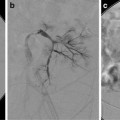
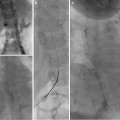
This 16-year-old had colon cancer discovered when he had a laparotomy after a motor vehicle accident. (a) Because of the scarring from the prior surgery, the surgeon did not want to resect the segment II hepatic metastasis (arrow) as noted on this contrast-enhanced fat-suppressed T1-weighted MR. (b) After RFA the left hepatic lesion was partially ablated. Note the hyperdense burn zone (short arrows) is not completely covering the hypodense lesion (long arrow) on this contrast-enhanced CT. (c) A sagittal US image shows the RF target (arrows) for re-ablation in the left lobe of the liver. (d) Because this lesion was so close to the esophagus, this procedure was done under fluoroscopy and US guidance. A triple (cluster) Cool-tip RF probe was placed. Two catheters were placed in the esophagus, which was only 8 mm away from the RF probe. One catheter was perfused with cold water and the other was placed to wall suction. This was done to protect the esophagus during RFA
The third group of malignant lesions in children that may be amenable to RFA are soft tissue or bone lesions. Examples include rhabdomyosarcoma in the breast and maxilla, leiomyosarcoma of the chest wall, and metastatic osteosarcoma to the humerus [1].
Contraindications
Contraindications are listed in Table 24.1.
Table 24.1
Contraindications to radiofrequency ablation
1. Proximity of intended target tumor to adjacent vital structures |
a. Tumor <1 cm from the main bile duct |
b. Tumor <1 cm from the portal vessel (without adjunct technique to minimize heat sink effect) |
c. Tumor <1 cm from the colon, small bowel, or stomach (without adjunct technique to displace) |
2. Intrahepatic bile duct dilatation (poor prognostic indicator) or presence of biliary-enteric anastomosis |
3. Exophytic tumor (risk of tumor seeding) |
4. Untreatable/unmanageable coagulopathy |
5. Lack of safe access route to tumor |
6. Active infection |
7. Pulmonary insufficiency (for lung RFA) |
Preprocedure Work-Up
Cross-sectional imaging with PET/CT is preferred prior to pulmonary RFA. MR of the head and neck, pelvic, or extremity lesion is preferred. MR (Fig. 24.1a) or CT (Fig. 24.1b) of hepatic or retroperitoneal lesions is sufficient. If US guidance is going to be used such as in hepatic (Fig. 24.1c), retroperitoneal, or soft tissue lesions, then US should be performed prior to the ablation by the interventionalist that will perform the ablation. A radiograph of a bone lesion is also necessary to rule out a pathological fracture.
A complete blood count, electrolytes, BUN, creatinine, liver function, and coagulation testing should be performed. Thrombocytopenia, anemia, coagulation disorders, and electrolyte derangements should be corrected. Pulmonary function testing should be performed prior to RFA of the lung.
A history and physical examination is performed in the clinic to include a pain intensity assessment. Consent during an unhurried clinic visit is warranted so the patient and family are fully aware of the risks and potential complications of this procedure.
Equipment and Procedure Technique
General anesthesia is necessary for all RF ablations in children. There are multiple types of RFA systems available that include mono- and bipolar devices. The control methods for energy deposition vary depending on the manufacturers. Common approaches include monitoring of impedance or temperature. It is important to be familiar with the specific control of your institutional RFA system.
The author is most familiar with using Radionics, ValleyLab, and Covidien (Boulder, CO) as progressive vendors for the same RF ablation system. A Cool-tip RF system has a 200 W 480 kHz generator with continuous internal cycling of chilled saline to minimize charring of tissues and to increase tissue impedance. It works using either manual control or impedance control. Grounding pads are placed transversely typically over the thighs. If a single RFA probe is used, two grounding pads are sufficient. If more probes are used, four grounding pads are necessary (anterior and posterior on each thigh). The linear 17 gauge RFA probes are cooled at the active tips. This prevents charring and gas formation and increases the burn volume by more effectively conducting the current to the periphery of the tumor. An impedance-controlled system also aids in conduction of the energy to a larger volume by shutting off the energy if the baseline impedance rises more than 20 Ω. The active tip length varies from 1 to 4 cm. For most malignant lesions, a cluster probe with three linear probes separated by 5 mm and with 2.5 cm active tips can be used (Fig. 24.1d). The probe is placed percutaneously under CT/CT fluoroscopy guidance for lung lesions and by US for most other lesions. The probe is placed in the deepest portion of the tumor. Typically the lesion is burned for 12 min, and then the energy and cold perfusion is turned off. A temperature of ≥60 °C at the tip of the probe indicates adequate tumor ablation in that zone. If, however, the temperature does not reach 60 °C, measures such as stopping cooling and impedance control can be used to reach a temperature of 90 °C for 1 min. Then the probe is pulled back to a location that registers 55 °C. If this is inside of the tumor, the cool perfused impedance-controlled ablation is repeated. If the 55 °C is outside the tumor, then the percutaneous tract is ablated by a series of manual burns avoiding burning the skin. To cover a larger burn volume, a switch controller allows three probes with 3 or 4 cm active tips to be placed farther apart and at different angles during a single ablation. The switch controller allows for each probe to be turned on sequentially and thus avoids the interference and incomplete ablation zones between probes. The ablation time with the switch controller is often 18 min. Having the manufacturer’s representative with you for your ablations is recommended until you are quite familiar with the technique.
Complications and Their Preventions
It is important to keep up to date on the complications of all thermal therapies. The US Food and Drug Administration (FDA) mandates reporting of all serious and fatal complications in the Manufacturer and User Facility Device Experience (MAUDE) database: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.cfm?searchoptions=1. To search for fatal complications of thermal ablations, enter: Event Type—Death and Product Code—GEI.
Significant complications such as fatal hemoptysis have been reported after pulmonary RFA. This MAUDE database revealed 19 deaths related to RFA, five related to pulmonary RFA, one included massive fatal hemoptysis. Two other cases of massive hemoptysis, 7 and 17 days post RFA, respectively, have been recently reported to be associated with pulmonary pseudoaneurysms treated successfully with coil embolization [3, 4]. In one large series, there was a 3 % incidence of procedural-related deaths after pulmonary RFA [5], with an increased risk of hemoptysis in patients receiving prior radiotherapy. Nonfatal hemoptysis can occur in 9 % of patients after RFA of lung lesions [6]. Treatment of nodules near large pulmonary arteries was thought to be safe but not effective [7]. One is taught that vessels over 4 mm in diameter are spared by RFA due to their internal cooling of normal blood flow. However, if the ventilation is compromised in the lung during RFA, pulmonary artery perfusion may decrease by as much as 84 % allowing larger pulmonary arteries to be thermally injured [8]. Delayed pseudoaneurysms and hemoptysis have been reported at 2 weeks post ablation of central pulmonary lesions. The lesions that involve the main or intermediate branch pulmonary arteries therefore should be avoided.
Left diaphragmatic herniation after pulmonary RFA in children has also been reported [1] after RFA of left lower lobe pulmonary metastases in contact with the diaphragm. RFA of lesions abutting the right diaphragm did not result in herniation possibly due to the large liver surface.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree