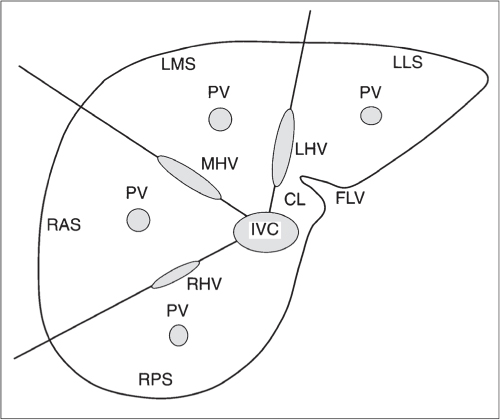
Vascular |
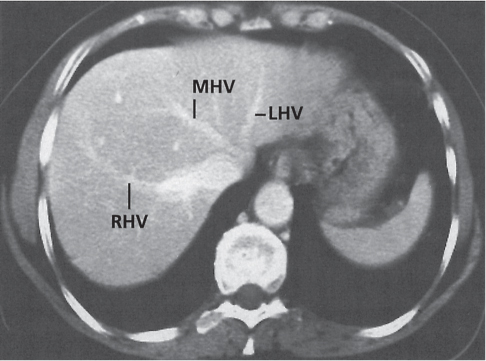
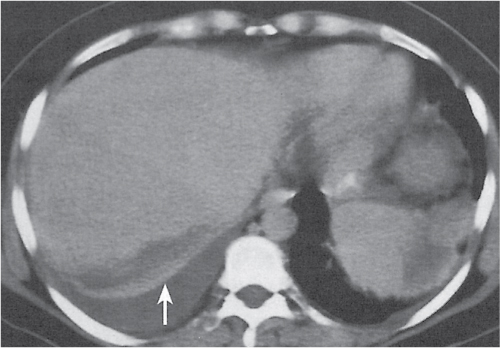
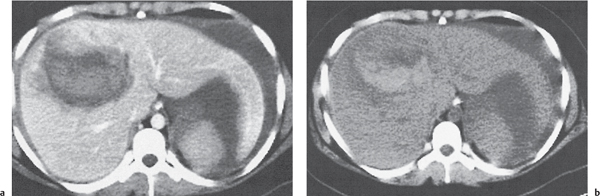
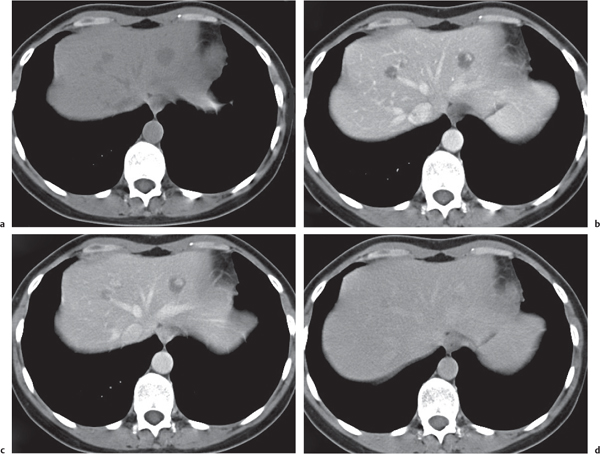
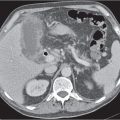
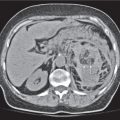
Hepatic venous outflow obstruction (Budd–Chiari syndrome)
Fig. 19.4 |
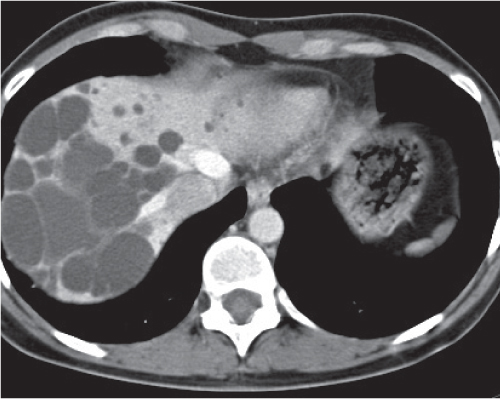
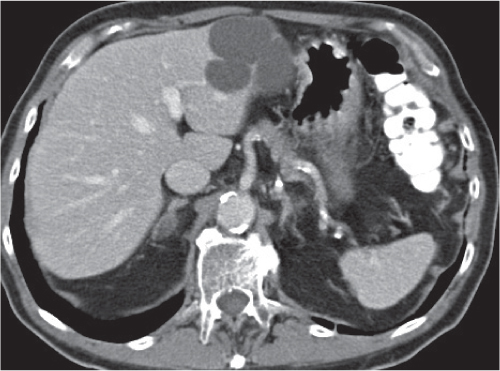
Segmental or global venous outflow obstruction. Often accompanied by large regenerative nodules. Hepatic veins usually not visualized or even clotted with thrombotic material (hypodense).
Scan recommendation:
Multiphasic CT (nonenhanced CT, portal venous phase [PVP], parenchymal phase [PP]). Nonenhanced CT: Typically, peripheral low attenuation of the liver parenchyma, with higher attenuation in central portions of the left lobe and the caudate lobe. PVP: Patchy enhancement on early contrast images. PP: Reverse of this pattern or persistent patchy central enhancement.
Diagnostic pearls: Despite appropriate enhancement of the inferior vena cava (IVC), aorta, and hemiazygos vein, there is a lack of contrast in the hepatic veins. Characteristics include collateral gastric veins “flip-flop” pattern of central portions of the left liver lobe and caudate lobe on PVP (enhancement) and PP images (decreased enhancement). |
Rare clinical entity of obstruction of hepatic venous outflow. Causing agents may be pregnancy, hyper-coagulopathy states, oral contraceptives, invading tumors, and congenital webs. Occlusion may occur at the level of intrahepatic venules, hepatic veins, or the IVC. Associated findings include narrowing of the IVC and dilated collateral veins (azygos, hemiazygos, or subcutaneous), ascites, and portal thrombosis. Hepatomegaly, regional intrahepatic attenuation differences, and large regenerative nodules are characteristic. |
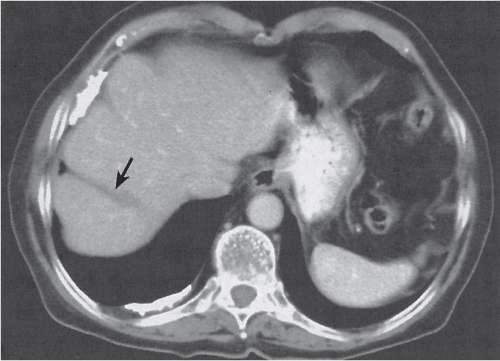
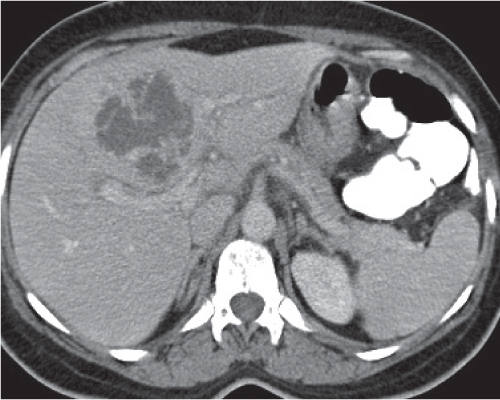
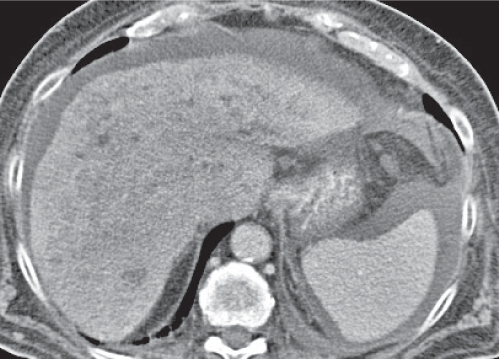
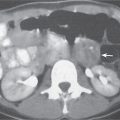
Liver infarction
Fig. 19.5 |
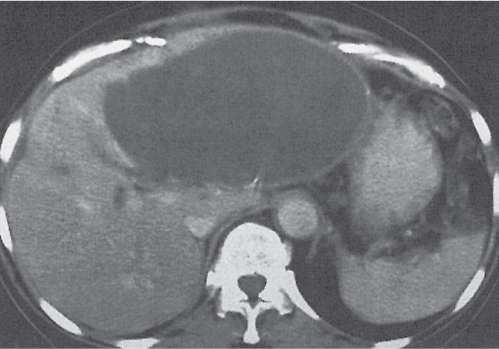
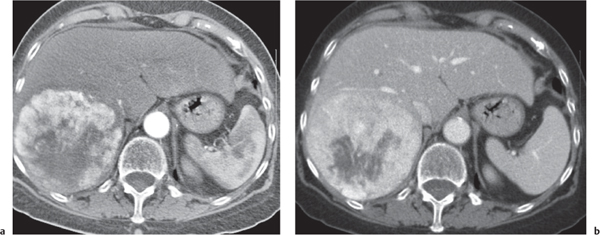
A wedge-shaped, low-attenuation lesion due to local vascular obstruction. Initially, infarcts are poorly marginated but become more discernible in a subacute/chronic situation.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Wedge-shaped, low-attenuation lesion with poor delineation to surrounding liver tissue. The more subacute/chronic the infarction, the better demarcated the margins.
PP: Distribution segmental or diffuse, non- or patchy heterogeneous enhancement, depending on degree and extent of occlusion.
Diagnostic pearls: Wedge-shaped, low-attenuation lesions with segmental distribution on pre- and postcontrast scans. |
Histologic feature of acute hepatic infarction is centrilobular necrosis with local hemorrhage. Chronic findings include focal atrophy of the involved segment and hypodense or cystic changes.
The portal venous system accounts for 75% of the blood supply of the liver. Thus, hepatic infarction usually is caused by the superimposition of portal vein occlusion on preexisting hepatic artery stenosis. Etiology for hepatic artery stenosis includes atherosclerosis, embolism, thrombosis, vasculitis, and hypotension/shock. Portal vein occlusion/thrombosis may be (1) caused by pregnancy or oral contraceptives, (2) iatrogenic (during intra-abdominal surgery, surgery of thrombogenic organs (prostate, uterus, etc.), or (3) paraneoplastic (pancreas). |
Infection |
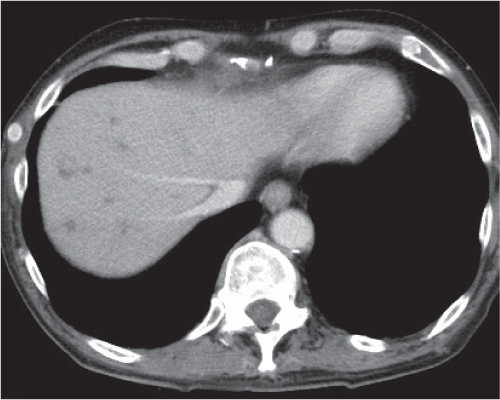
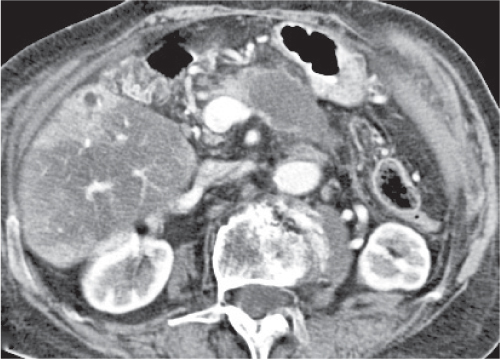
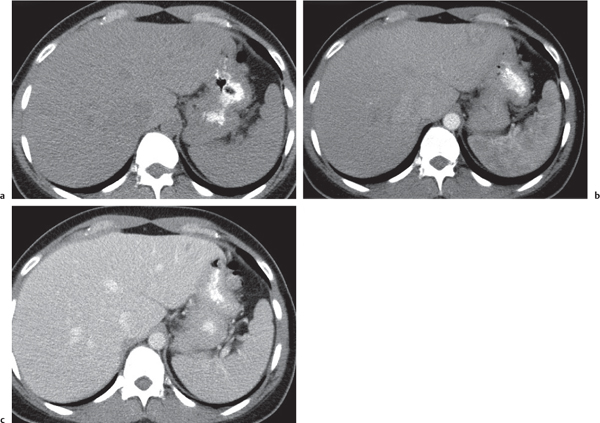
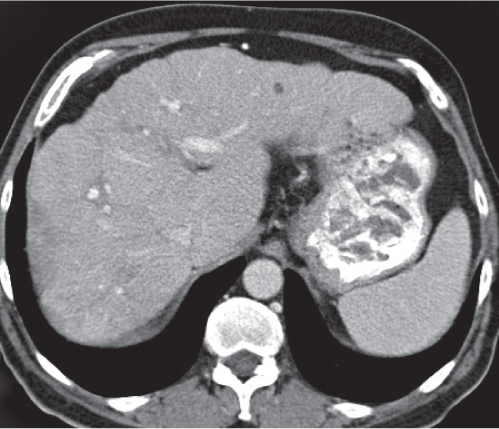
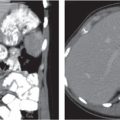
Pyogenic abscess
Fig. 19.6 |
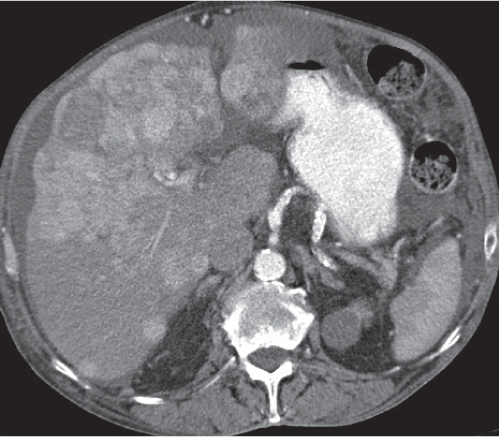
Small abscesses are sharply defined lesions, often surrounded by a capsule and septated. They may aggregate into a single large cavity.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Well-defined, round lesions, denser than water but less dense than liver.
PP: Distinct enhancement of capsule and septa. No enhancement of central portion (necrosis).
Diagnostic pearls: “Cluster of grapes” sign: cluster of multilocular collections of pus coalescing into larger centrally located septated cavities. |
Destruction of hepatic parenchyma with localized collection of pus due to a bacterial infection. Twenty to 30% contain gas, but gas–fluid levels are rare. Immature abscesses may not be cystic. Usually occur in immunocompromised or older patients with predisposing conditions, such as biliary/pancreatic diseases, diverticulitis, colitis, appendicitis, trauma, and septicemia. Necrotic metastases (from sarcoma or ovarian carcinoma) may look alike and even become infected. |
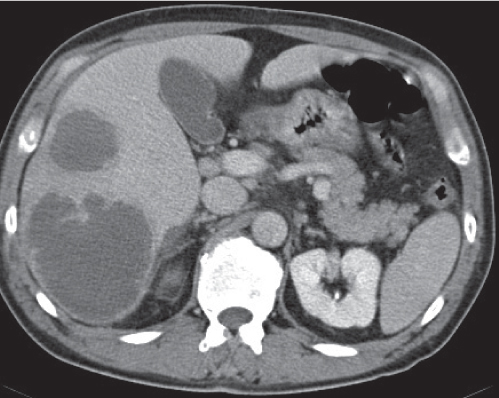
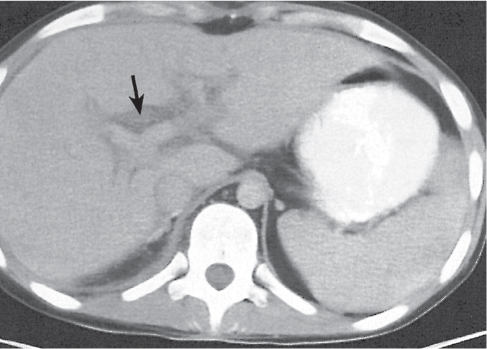
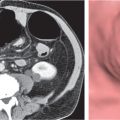
Opportunistic abscess
Fig. 19.7 |
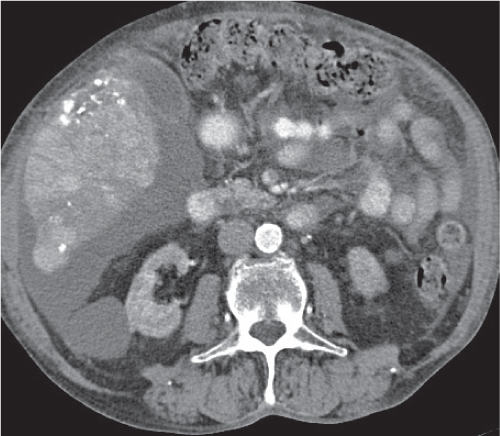
Multiple round hypodense hepatic lesions. Micronodular abscesses may even make liver appear almost normal or slightly heterogeneous.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Multiple small hypodense lesions.
PP: Lesions remain hypodense without any attenuation.
Diagnostic pearls: Multiple ill-defined micronodular hypodense lesions seen on pre- and postcontrast scans. |
Microabscesses typically are observed in immuno-compromised patients and are caused by Candida, less commonly by Aspergillus, Pneumocystis carinii, or atypical mycobacteria. Because needle biopsy may be false-negative, open wedge biopsy is usually required for final diagnosis.
Differential diagnosis: rule out biliary hamartomas, Caroli disease, and metastases. Typically, similar nodules are also found in the spleen and/or lung (septicemia). |
Amebic abscess
Fig. 19.8 |
Peripherally located, well-defined, round, hypodense cystic lesion with enhancing capsule.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Hypodense as compared with normal liver parenchyma.
PP: Nonenhancing center, enhancing inner rim, and hypodense outer rim.
Diagnostic pearls: Well-defined, round, targetlike lesions with preference for the right liver lobe. |
Underlying infection is amebiasis of the cecum and ascending colon. In ~8% of cases, amebas invade liver through the portal venous system.
Common in developing countries (~10% of the world population). May be complicated by rupture into the peritoneum, through the diaphragm, subphrenic abscess, biliary obstruction, or septic emboli. Do not touch lesions; antimicrobial therapy and cytotoxic drugs are treatment of choice.
Differential diagnosis: rule out hepatic pyogenic abscess, hepatic hydatid cyst, and biliary cystadeno-carcinoma. |
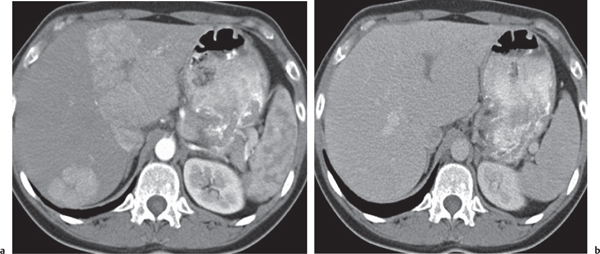
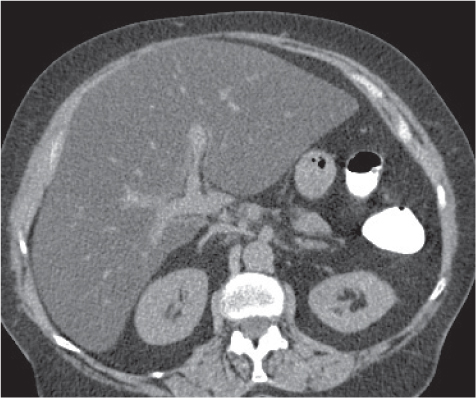
Echinococcal (hydatid) cyst
Fig. 19.9 |
Large, well-defined, cystic liver mass surrounded by several peripheral satellite (daughter) cysts.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Cysts appear hypodense to normal liver parenchyma.
PP: Enhancement of cysts’ walls and septations.
Diagnostic pearls: Large well-defined, hypodense, cystic liver mass with intracystic septations, irregular wall calcifications surrounded by numerous (less hypodense) daughter cysts. |
Caused by Echinococcus granulosus or Echinococcus multilocularis: parasitic eggs are ingested after contact with dogs or foxes/contaminated food or water. E. granulosus infiltrates liver within days, develops into typical hydatid cysts, and grows up to 3 cm per year in diameter.
E. multilocularis effects a diffuse tissue infiltration, induces a granulomatous hepatic tissue reaction followed by necrosis, cavitation, and calcifications. Air–fluid levels typically represent cyst rupture and not infection. Rupture into the biliary tree is the most common complication, causing biliary dilation and cyst wall discontinuity. Clinical symptoms of biliary rupture are pain, fever, and jaundice. Treatment of choice is surgical resection; alternatively, percutaneous aspiration/injection and albendazole may be used. |
Congenital |
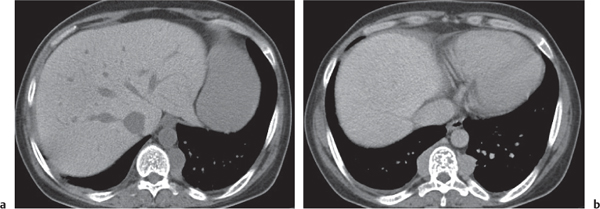
Caroli disease
Fig. 19.10a, b |
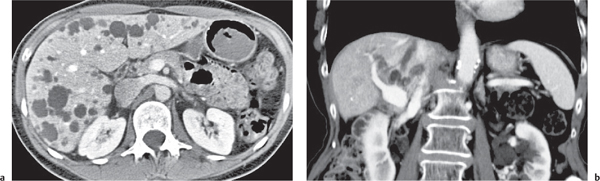
Multiple low-attenuating cystic masses in the liver associated with a dilated biliary tree.
Scan recommendation:
Biphasic CT (nonenhanced CT, PVP, PP).
Nonenhanced CT: Multiple round to saccular hypodense cysts.
PP: Cysts may contain a hyperdense central “dot” representing enhancing portal radicles.
Diagnostic pearls: Multiple well-delineated, round to saccular hypodense cysts with a hyperdense central “dot” during the PVP. |
Rare condition characterized by multiple saccular dilations of the intrahepatic bile ducts throughout the liver. Medullary sponge kidney is associated in 80% of cases. |
Trauma |
Biloma (bile pseudocyst)
Fig. 19.11 |
Water-density, usually crescent or ovoid mass within or immediately adjacent to the liver.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Near-water density.
PP: No contrast enhancement of the wall or contents.
Diagnostic pearls: Well-delineated, homogeneous, round, near-water- density lesions without enhancement after contrast application. |
Intra- or extrahepatic collection of bile after traumatic rupture or surgery of the biliary tree. |
Laceration
Fig. 19.12 |
Irregular cleft or low-attenuation area that often extends to the periphery of the liver. A solitary (simple) or branching (stellate/radiating pattern may be seen. Often accompanied by intra- and perihepatic hemorrhage.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Lacerations show near-water density. Hemorrhage may be acute (unclotted) or subacute (clotted). Unclotted blood (> 50 HU) and clotted blood (< 90 HU) typically are hyperdense to lacerations and liver parenchyma.
PP: Active hemorrhage seen as contrast extravasation (Caveat: Injection rate must be ≥ 5 mL/s). |
Small hyperdense blood clots may be detected within the laceration. Lacerations evolve from sharply marginated low-attenuation lesions into indistinguishable areas after several weeks. |
Intrahepatic hematoma
Fig. 19.13a, b |
Round, oval, changing density patterns depending on acuteness of hemorrhage.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Subacute hematoma usually hyperdense (< 90 HU), chronic hematoma (10–30 d after onset of bleeding) usually hypodense (20–25 HU).
PP: Subacute hematoma may become isodense after CM.
Diagnostic pearls: Hyperdense intrahepatic round, oval, or irregular lesions on nonenhanced CT scans. |
Usually secondary to penetrating or blunt trauma, rarely due to adenoma, metastasis, or arteriovenous malformation (AVM).
Differential diagnosis: bilioma: remains hypodense on all scans. |
Subcapsular hematoma |
Well-marginated, crescentic or lenticular fluid collection beneath the hepatic capsule. High-attenuation lesion in the beginning and diminishing gradually over several weeks to become a low-attenuation lesion.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Subacute hematoma usually hyperdense (< 90 HU), chronic hematoma (10–30 d after onset of bleeding) usually hypodense (20–25 HU).
PP: Subacute hematoma may appear isodense after CM.
Diagnostic pearls: Well-marginated, crescentic fluid collection beneath the hepatic capsule. |
May result from trauma, surgery, or percutaneous interventions (cholangiography, biopsy, portography, or biliary drainage procedures). Patients usually report right chest pain during breathing or pain radiating into the right shoulder. Pain usually diminishes within a few days, but it may continue. Keep patients on strong medication as needed. |
Inflammation |
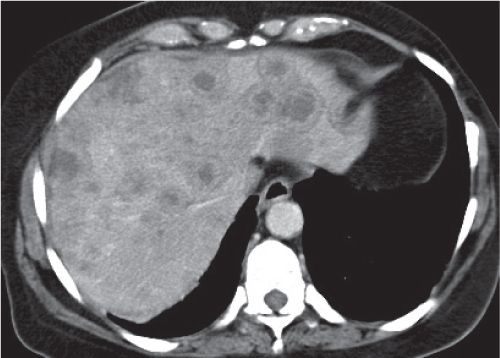
Hepatic sarcoidosis
Fig. 19.14 |
Presence of diffuse small noncaseating granulomas in the liver of patients with known Boeck disease (sarcoidosis).
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT/PVP: Multiple ill-defined, hypodense hepatic noduli or heterogeneous appearance of liver parenchyma, often accompanied by hepatosplenomegaly.
PP: Hepatic noduli become isodense to normal liver parenchyma.
Diagnostic pearls: Always consider in patients with lymphadenopathy and hepatosplenomegaly. Often also found in the spleen. |
Histologically, noncaseating epithelioid granulomas with multinucleated giant cells of Langerhans type. Causing agent of granulomas unknown.
Two thirds of patients are women 20 to 40 y old. Up to 80% of all patients with sarcoidosis present with involvement of abdominal organs, particularly of the liver and spleen. Treatment through corticosteroids, antiinflammatory agents, and cytotoxic drugs.
May not be discernible from liver hamartoma, opportunistic abscesses, metastases. |
Focal fatty infiltration
Fig. 19.15 |
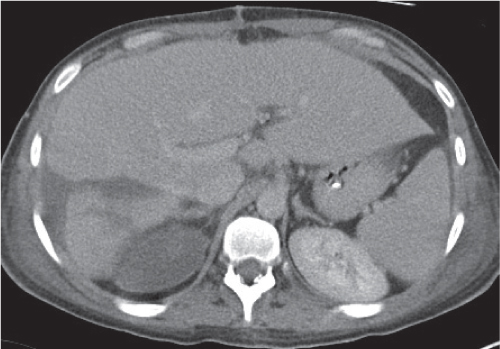
Varies from a small oval focus, typically adjacent to the intersegmental fissure, to segmental, lobar, or geographic distribution. No mass effect, contour change, or vascular displacement.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Poor delineation to normal liver parenchyma (iso-/slightly hypodense).
PP: Slightly hypodense as compared with normal liver parenchyma; noncompromised intralesional vessels.
Diagnostic pearls: Poorly delineated lesion often adjacent to the intersegmental fissure slightly hypodense to normal liver parenchyma on all phases and noncompromising hepatic vessels. |
Histologically regional reversible accumulation of triglycerides within hepatocytes that can simulate neoplasia. Typically associated with obesity, alcoholism, small bowel bypass surgery, diabetes, chemotherapy, hyperalimentation, blunt abdominal trauma, cystic fibrosis, congestive heart failure, Reye syndrome, steroid therapy, and Cushing syndrome. Lesion may occur and disappear within weeks. |
Periportal edema
Fig. 19.16 |
Nonenhancing low-density collection encasing the branches of the portal vein (see also Fig. 24.3 , p. 741). |
Common postoperative finding in liver transplants. Does not indicate rejection. May also occur in combination with significant edema of other abdominal organs. |
Radiation hepatitis |
Sharply defined band of diminished density in the liver corresponding to the radiation port. |
May develop within days/months after radiation therapy (radiation dose ≥ 35 Gy). May show evidence of recovery. |
Benign neoplasms |
Hepatic hemangioma
Fig. 19.17a–d |
Benign neoplasm of thin, fibrous stroma that surrounds multiple vascular channels lined by a single layer of endothelial cells.
Scan recommendation:
Multiphasic CT (nonenhanced CT, hepatic arterial phase [HAP], PP, delayed phase [DP]).
Nonenhanced CT: Small hemangiomas are usually well-circumscribed masses isodense to blood. Large hemangiomas appear more heterogeneous and may contain central fibrotic cleft of low density.
HAP: Early peripheral global or nodular enhancement.
PP: Progressive centripetal filling usually isodense to blood.
DP: Persistent global enhancement that stays isodense with blood.
Diagnostic pearls: < 10 cm lesion commonly in subcapsular location (right > left liver lobe) with peripheral globular enhancements on HAP scans and progressive centripetal enhancement following contrast phases (flash filling). Hemangiomas usually are isodense to blood and thus show a similar enhancement pattern as the aorta. |
The most common hepatic neoplasm, seen in 7% or more of the normal population. A significant proportion of hemangiomas does not present a characteristic pattern of contrast enhancement and may thus simulate metastasis.
Metastases from breast carcinoma and gastrointestinal stromal tumor (GIST) may not be discernible from typical hemangiomas (see Fig. 24.13 , p. 747). Hemangiomas grow slowly from childhood to adulthood, more rapidly during pregnancy. May spontaneously regress. |
Focal nodular hyperplasia (FNH)
Fig. 19.18a–c
Fig. 19.19a, b |
Well-circumscribed benign hamartomatous liver lesion with subcapsular preference. Majority of FNH appear solitary and < 5 cm in diameter (80% of cases).
Scan recommendation:
Multiphasic CT (nonenhanced CT, HAP, PP, DP). Nonenhanced CT: Hypo- or isodense to normal liver parenchyma. Any central scar appears even more hypodense.
HAP: Bright homogeneous enhancement.
PP: Hypo-/isodense to normal liver parenchyma.
DP: Isodense to normal liver parenchyma. If there is a scar, it is hyperdense.
Diagnostic pearls: Well-differentiated mass with distinct homogeneous enhancement on HAP and continuously becoming isodense to liver parenchyma; on delayed images, often showing a hyperdense central scar. May resemble a cross section of a grapefruit. |
Rare benign liver tumor most often seen in women between age 20 and 50 y.
Histologically, a hyperplastic stroma response to local abnormal vasculature. Oral contraceptives have stimulating effect on FNH growth but are not responsible for existence per se. Usually incidentally detected, multiple in 7% to 20% of cases.
Central scars are visible in ~70% of large and 30% of small FNHs. Central scar also seen in atypical HCCs, fibrolamellar hepatomas, hepatic adenomas, and giant hemangiomas. Absence of intratumoral calcifications, necrosis, and hemorrhage may help to differentiate from these lesions. Any definitive diagnosis requires hepatic biopsy. |
Hepatic adenoma
Fig. 19.19a, b |
Heterogeneous subcapsular hypervascular lesion, on average 8 to 15 cm in diameter, with a preference for the right lobe of the liver and surrounded by a pseudocapsule.
Scan recommendation:
Multiphasic CT (nonenhanced CT, HAP, PVP, DP). Nonenhanced CT: Well-defined mass with intratu-moral presence of hypodense (fat) and/or hyperdense (calcifications, hemorrhage) areas.
HAP: Heterogeneous hyperdense enhancement.
PVP: Hypo-/iso-/hyperdense to normal liver parenchyma.
DP: Homogeneous isodense (to liver) enhancement.
Pseudocapsule appears hyperdense as compared with normal liver parenchyma and adenoma.
Diagnostic pearls: Well-defined, heterogeneous (intratumoral fat/hemorrhage), hypervascular lesion with pseudocapsule on DP images in a noncirrhotic liver. |
Usually unilocular large lesions, typically in young women taking oral contraceptives. May also occur in men who use androgens or anabolic steroids. Multilocular adenomas (hepatic adenomatosis) seen in patients with glycogen storage disease or diabetes mellitus. Internal hemorrhage or necrosis is common (36%). Histologically, adenomas lack bile ducts and portal/central veins but contain hepatocytes that are arranged in cords/sheets. May contain malignant foci. Forming of a pseudocapsule due to compression of adjacent liver tissue. On CT scans, often indistinguishable from HCC, but presence of a pseudocapsule and absence of cirrhosis are suggestive. |
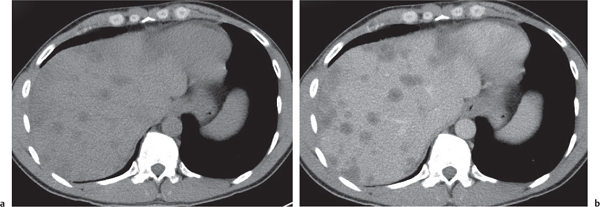
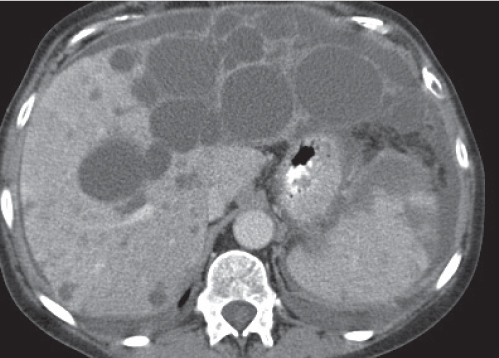
Nodular regenerative hyperplasia
Fig. 19.20 |
Either multiple micronodular or solitary macronodular, preferably hypervascular liver lesions. Size varies from < 1 mm (micronodular) to 4 cm (macronodular).
Scan recommendation:
Multiphasic CT (nonenhanced CT, HAP, PVP).
Nonenhanced CT: Lesions isoattenuating to normal liver parenchyma.
HAP/PVP: Homogeneously hyperdense lesions sometimes with hypodense peripheral “halo.”
Diagnostic pearls: Multiple micronodular liver lesions with hyperattenuation on both HAP and PVP images. |
Histologically, regenerative nodules consist of hyper-plastic hepatocytes surrounding dilated, atypically formed, large sinusoids. Peripheral halo, if present, evoked by enlarged surrounding sinuses (peliosis). May mimic metastases. Larger nodules may have central scar and thus be indistinguishable from FNH. |
Mesenchymal (biliary) hamartoma
Fig. 19.14 , p. 681 |
Multiple micronodular (< 15 mm), low-density cystic or solid lesions.
Scan recommendation:
Multiphasic CT (nonenhanced CT, PVP, PP).
Nonenhanced CT: Cystic lesions with near-water density. Solid lesions have low density compared with normal liver parenchyma.
PVP: Cystic lesions isodense to water; solid lesions enhance strongly.
PP: Cystic lesions remain isodense; solid lesions become isodense to normal liver parenchyma.
Diagnostic pearls: Multiple small lesions with identical near-water attenuation on pre- and postcontrast scans. |
Rare. Histologically, proliferated bile ducts embedded in mesenchymal tissue. May be associated with polycystic liver disease.
Solid lesions may mimic hemangioma.
Cystic lesions have similar CT features as sarcoidosis but without coexistent lesions in the spleen. |
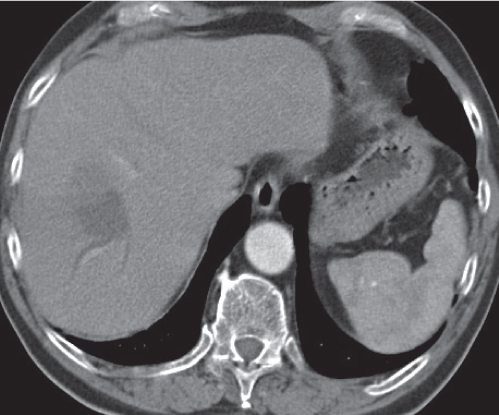
Hepatic cyst
Fig. 19.21 |
Sharply delineated, round or oval lesion of near-water attenuation.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Near-water density.
PP: No contrast enhancement of the wall or contents.
Diagnostic pearls: Well-delineated, homogeneous, round, near-water density lesions without enhancement after contrast application. |
May be multiple. Incidence is ~2.5% in the Western population, higher in Asian women. Associated with von Hippel–Lindau disease, polycystic kidney disease, and autosomal dominant polycystic liver disease.
In case of septations, irregular inner margins, intracystic components, and contrast attenuation rule out cystic neoplasm. Nonenhanced CT helps to distinguish intracystic hemorrhage (hyperdense) from neoplasm (hypodense). |
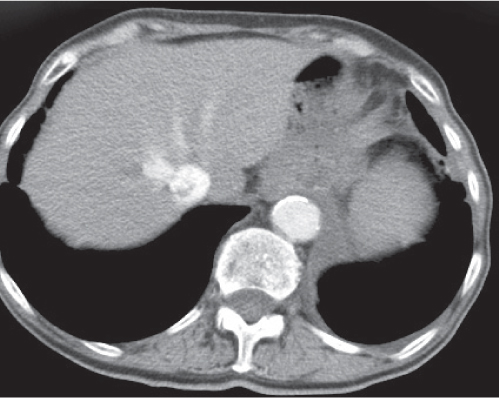
Hepatic angiomyolipoma
Fig. 19.22a, b |
Sharply delineated hepatic mass of variable shape and diameter (0.5–30 cm) containing intralesional fat.
Scan recommendation:
Multiphasic CT (nonenhanced CT, PVP, PP).
Nonenhanced CT: Heterogeneous attenuation depending on predominant tissue component.
PVP: Strong attenuation (angioid components).
PP: Delayed enhancement.
Diagnostic pearls: Well-delineated hepatic mass with hyperattenuation on PVP scans and presence of intralesional fat and vessels. |
Rare. Seen predominantly in patients with renal angiomyolipomas. Histologically, composed of fat, smooth muscle cells, and proliferating blood vessels. |
Biliary cystadenoma |
Cystic mass in the liver parenchyma with septated, multilocular appearance. |
Rare lesion, primarily in middle-aged women. May recur and develop into a malignant cystadenocarcinoma. Distinction not important as surgical resection is treatment of choice for both.
Differential diagnosis: liver abscess/hydatid cysts. |
Hepatic lipoma |
Distinct area of intrahepatic fat. Sometimes fine septa of soft tissue density are present. Identical low-attenuation values on pre- and postcontrast scans. |
Rare. May be distinguished from focal steatosis (poorly discernible, blood vessels crossing through lesions) and metastases from liposarcoma (heterogeneous lesions with distinct attenuation on postcontrast scans). |
Intrahepatic extension of a pancreatic pseudocyst |
Round intrahepatic cystic mass or tubular lucencies simulating dilated bile ducts (see also Fig. 20.11 , p. 707). |
Complication of pancreatitis. |
Malignant neoplasms |
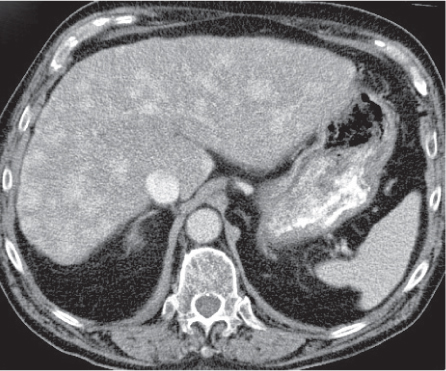
Hepatocellular carcinoma (HCC)
Fig. 19.23 |
CT appearance is variable: focal, multinodular, or diffuse. May contain fatty tissue, necrosis, and, occasionally, calcifications. Portal vein invasion in 25% to 40%, hepatic vein invasion in up to 15%.
Scan recommendation:
Triphasic CT (nonenhanced CT, HAP, PVP). Nonenhanced CT: Hypodense mass with or without necrosis, calcification, fat.
HAP: Heterogeneous wedge-shaped enhancement (perfusion abnormalities due to portal vein occlusion). PVP: Hypo isodense to liver parenchyma with local contrast accumulation.
Diagnostic pearls: Large heterogeneous, hypervascular mass invading portal vein suspicious for HCC. Smaller HCC may mimic metastasis/hemangioma in cirrhotic liver. |
The most common primary liver tumor. Predisposing conditions: cirrhosis due to ethanol or virus hepatitis, hemochromatosis, Wilson disease, Gaucher disease, glycogen storage disease (type 1), tyrosinosis, and biliary atresia. The total number and location of lesions are often difficult to estimate. |
Fibrolamellar hepatocellular carcinoma (HCC)
Fig. 19.24 |
Well-defined, large, solitary heterogeneous mass; often hypodense central scar and radial septa. Necrosis and calcification are common (30% of patients).
Scan recommendation:
Multiphasic CT (nonenhanced CT, HAP, PVP, DP). Nonenhanced CT: Well-defined hypodense mass. Necrosis and calcification are common.
HAP: Hyperattenuating and heterogeneous.
PVP: Hypo-/isodense to liver parenchyma.
DP: Hyperdense scar/septa/capsule. |
Rather uncommon form of HCC. Involves younger population and is unrelated to cirrhosis. Less malignant than standard HCC. Lymph node/lung metastases and biliary/vessel infiltration are signs of malignancy.
May mimic FNH/HCC. Central scar on T2-weighted MRIs hyperintense in FNH versus hypointense in fibrolamellar HCC. HCC lacks scar and often comes with underlying cirrhosis. |
Hepatic metastases
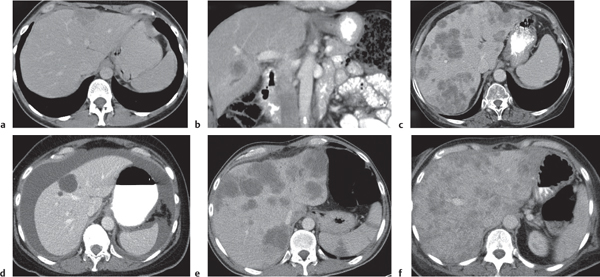
Fig. 19.25a–f |
Either hypo- (common) or hyperdense (less common) lesions with random distribution pattern within normal-enhancing liver tissue. Variable in size (few millimeters to several centimeters).
Scan recommendation:
Triphasic CT (nonenhanced CT, HAP, PP) necessary only for initial oncological staging.
Nonenhanced CT: Iso-/hypoattenuating to normal liver parenchyma.
HAP: Hypervascular metastases appear hyperdense.
PP: Hypodense, sometimes hypodense center (central necrosis) with peripheral rim enhancement (viable tumor or compressed normal parenchyma).
Diagnostic pearls: Hepatic cysts, hemangiomas, abscesses, sepsis, sarcoidosis, peliosis hepatis, and biliary hamartoma may mimic metastases. Vascular rim enhancement typical for epithelial metastases. Metastases of ovarian cystadenocarcinoma typically infiltrate liver from outside and thus may be found primarily in a subcapsular location. Breast cancer metastases may be indistinguishable from hemangiomas. |
Most common liver malignancy (~20 times more common than all primary liver neoplasms combined). Common sites of origin are the colon, stomach, pancreas, breast, and lung. Usually multiple and involving both lobes. Right or left lobe involvement occurs in 20% and 3%, respectively. New appearance of a liver lesion in a patient with a known malignancy most indicative of a metastatic lesion. Pre- and early postcontrast scans are recommended to depict the maximum number of metastases. CT features lack histologic specificity except for vascularity. Pancreatic islet cell, carcinoid, renal cell carcinoma, sarcoma, pheochromocytoma, and germ cell tumors often are hypervascular.
Cystadenocarcinoma and sarcoma (pancreas, GI, ovarian) often cystic (hypoattenuating). Mucinous carcinomas (colon, rectum, and stomach), treated breast, medullary thyroid, osteosarcoma, carcinoid, and leiomyosarcoma metastases frequently calcify. |
Hepatic lymphoma
Fig. 19.26 |
Primary hepatic lymphomas (rare) or secondary involvement in Hodgkin disease or non–Hodgkin lymphoma.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT necessary only for initial oncological staging.
Diagnostic pearls: Primary liver lymphoma is a solitary mass with no distinguishing CT features. Secondary infiltrating lymphoma is usually impossible to differentiate from normal liver parenchyma. Nodular or mixed forms are often low attenuating and distinguishable on CT but less common. |
Due to a high content of lymphatic tissue, lymphomas are often found in the periportal area. Primary lymphoma of the liver is rare, but secondary lymphomatous involvement is found in 60% of Hodgkin disease and in 50% of non–Hodgkin lymphomas. May complicate liver transplant. |
Hepatic sarcomas (angiosarcoma, hemangiosarcoma, and hemangioendothelial sarcoma) |
Hemorrhagic, hypervascular, heterogeneous tumor: multifocal or diffusely infiltrating. Size ranging from micronodular to large. Vascular channels within lesion may be capillary or cavernous.
Scan recommendation:
Triphasic CT (nonenhanced CT, PVP, DP) shows progressive enhancement over time.
Nonenhanced CT: Single/multiple hypodense masses. Central hyperdensity represents fresh hemorrhage.
PVP: Usually hypodense mass with heterogeneous nodular enhancement. Rarely peripheral nodular enhancement with centripetal progression.
DP: Persistence of contrast material in central vessels.
Diagnostic pearls: Multiphasic CT shows progression of contrast enhancement over time. Consider hemangiomas as differential diagnosis that usually enhance relative to blood vessels (aorta, vena cava). |
Most common mesenchymal liver tumor (~2%) often associated with liver cirrhosis, hemochromatosis, or von Recklinghausen disease. Histologically, a malignant spindle cell tumor of endothelial origin, forming poorly organized vessels and growing along predefined vascular structures. Typically also involves skin, soft tissue, breast, liver, and spleen. Environmental carcinogens (such as thorotrast, polyvinyl chloride, etc.) and radiation exposure may trigger tumor growth. |
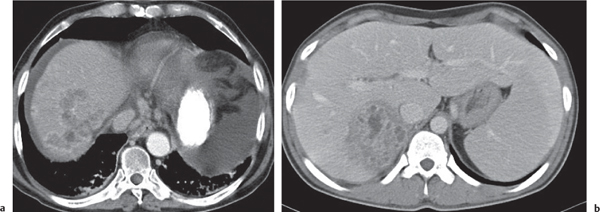
Epithelioid hemangioendothelioma
Fig. 19.27a, b |
Well-defined, peripheral (often confluent), typically targetlike nodules. Calcifications rare.
Scan recommendation:
Multiphasic CT (nonenhanced CT, PVP, DP).
Nonenhanced CT: Nodules of decreased attenuation as compared with normal liver.
PVP/PP: Targetlike appearance: Hypodense center (myxoid stroma); hyperdense inner peripheral rim (highly vascularized); hypodense outer peripheral rim (edema).
Diagnostic pearls: Targetlike appearance of peripherally located and partly coalescing lesions. Capsular retraction due to fibrosis and ischemia. |
Low-malignant, slowly progressing, highly vascularized liver tumor of unknown etiology. Epithelioid cells stain positive for factor VIII–related antigen. Tumor affects women more than men (association with contraceptives?). Typical age group: 28 to 58 y. Eighty percent of patients survive 5 to 10 y after diagnosis. Nodule resection and liver transplantation are treatment of choice. |
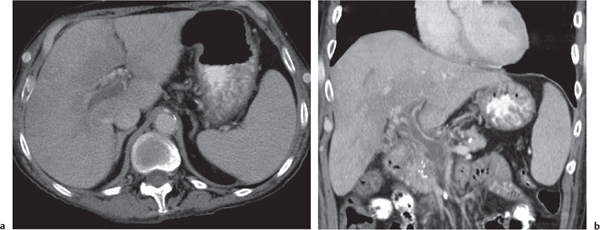
Peripheral (intrahepatic) cholangiocarcinoma (PCC)
Fig. 19.28a, b |
Heterogeneous large hepatic mass with dilated bile ducts and capsular retraction. Can be mass forming (well circumscribed), periductal infiltrating (i.e., branchlike growth along bile ducts with local obstruction of bile ducts), or intraductal growing (segmental/focal bile duct dilation).
Scan recommendation:
Multiphasic CT (nonenhanced CT, HAP, PP, DP).
Nonenhanced CT: Well-defined, lobulated, hypodense mass often with punctuated calcifications and intrahepatic bile duct dilation.
HAP: Rimlike enhancement.
PP: Progressive centripetal filling usually not isodense to blood.
DP: Distinct delayed enhancement (due to fibrotic stroma of tumors)
Diagnostic pearls: Tumor often visible only on delayed images. Tumor margins often best depicted on DP/PP images. |
PCC and intraductal growing cholangiocarcinoma often missed due to ill-defined margins and low attenuation. Five to 10 minutes after contrast injection is optimal for delayed imaging.
Accounts for ~15% of liver cancers, but only 10% of all cholangiocarcinomas are intrahepatic. Associated with repeated cholangitis, hepatolithiasis, congenital cystic liver disease, and primary sclerosing cholangitis (PSC). Age peak: 50 to 60 y. Treatment of choice is surgical resection. Five-year survival rate is 30%. |
Hepatoblastoma |
Low-attenuation hepatic tumor with peripheral rim enhancement.
Scan recommendation:
Consider MRI instead of CT (radiation issues with children). |
Most frequent hepatic malignancy in children, usually affecting the right lobe. |
Embryonal sarcoma |
Large liver tumor with numerous cystic regions.
Scan recommendation:
Consider MRI instead of CT (radiation issues with children). |
Rare tumor found exclusively in pediatric patients; 75% occur in the right lobe. |
Biliary cystadenocarcinoma
Fig. 19.29 |
Large, well-defined, complex cystic mass with macroscopic nodules, septations, and, rarely, calcifications. |
Eighty-five percent of tumors arise from intrahepatic bile ducts, 14% from extrahepatic bile ducts, and < 1% from the gallbladder.
Distinction between (benign) cystadenoma and cyst-adenocarcinoma not important as surgical resection is treatment of choice for both. |
Extrahepatic tumors |
Tumors of adrenal, kidney, stomach, and gallbladder can invade or displace the liver and thus have an appearance similar to that of primary liver tumors. |
Multiplanar reformations allow for better delineation of anatomical structures/pathology. |