29 Pelvis
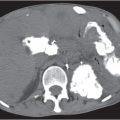
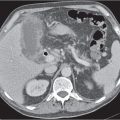
An understanding of soft tissue and bony pelvic anatomy on computed tomography (CT) aids in the detection of pelvic diseases and recognition of their patterns of spread. Nearly all major organ systems are represented in the pelvis, including the gastrointestinal (GI), genitourinary, central and peripheral nervous, angiolymphatic, and musculoskeletal systems. The limits of the pelvis are defined by the innominate bones, sacrum, and coccyx. The internal and external pelvic muscular and bony frameworks are relevant, as disease processes arising in these structures sometimes manifest within the pelvic cavity (Figs. 29.1 , 29.2).
The pelvic peritoneal reflections delineate significant but not insurmountable boundaries between extraperitoneal structures and the peritoneal cavity. The peritoneal reflections over the pelvic organs, vessels, and ligaments define several peritoneal pelvic cavity recesses, the largest and most gravity-dependent of which is the rectovesicle pouch. The rectovesicle pouch, along with the pelvic portion of the greater omentum, is one of the most common sites of tumor implants in peritoneal carcinomatosis.
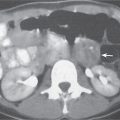
Pelvic peritoneal reflections often form anatomical landmarks recognizable on CT images where they envelop organs and the associated vascular and lymphatic systems. The peritoneal reflection forming the sigmoid mesocolon encompasses a portion of the proximal rectum and sigmoid colon, along with their associated angiolymphatic structures. The sigmoid mesocolon is more easily seen in the presence of ascites (Fig. 29.3). In addition, the small bowel and its mesentery, as well as their associated pathologic processes, often extend into the pelvis (Fig. 29. 4 ).
In women, the broad ligament is that part of the peritoneal reflection surrounding the margins of the uterus, fallopian tubes, and proximal round ligaments extending to the pelvic sidewall. The ovary is attached to the posterior broad ligament by the mesovarium. The peripheral broad ligament from the ovary to the pelvic sidewall that contains the ovarian vessels is referred to as the suspensory ligament of the ovary. The uterus divides the rectovesical pouch into the anterior vesicouterine pouch and the posterior rectouterine pouch, also known as the cul-de-sac or pouch of Douglas.

Although the remaining pelvic structures, such as the rectum, anus, distal ureters, bladder, cervix, vagina, seminal vesicles, prostate, and sidewall structures, do not readily protrude into the pelvic peritoneal cavity, they are often associated with fascial planes that frequently confine pathologic processes. These boundaries localize lesions to their site of origin, guiding the differential diagnosis of a pelvic mass.
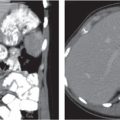
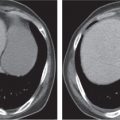
Ectopic organs, such as pelvic kidneys (Fig. 29.5), are readily identified on CT. Pelvic testicles present as elliptical foci along the course of the testicles’ descent, frequently within the inguinal canal (Fig. 29.6).
Some surgical procedures may result in pseudolesions due to the displacement of normal pelvic structures. In particular, abdominoperineal resection of the rectum can result in a posterior presacral position of the uterus in women or the prostate and seminal vesicles in men (Fig. 29.7). These normal presacral masses must be distinguished from presacral recurrent tumor and postoperative fibrosis. If a suspicious mass is encountered, biopsy is warranted. If there is question that a presacral mass is a displaced normal structure such as the seminal vesicles or uterus, magnetic resonance imaging (MRI) is often helpful.
Midline or lateral surgical transposition of the ovaries in women can also be problematic if the history of this procedure is not elicited, especially if there is a superimposed functional cyst. Lateral surgical ovarian transposition procedures are sometimes performed to preserve ovarian function in young women with cervical carcinoma who will receive radiation therapy. The ovaries are placed in the iliac fossae or high in the lateral abdomen (Fig. 29.8). Midline ovarian transposition may be performed in young women who will receive pelvic nodal radiation for lymphoma.
Other iatrogenic “pseudolesions” are intestinal urinary reservoirs in patients with urinary diversions, tissue expanders to displace bowel from radiation ports prior to radiation therapy (Fig. 29.9), and implanted reservoirs for penile prostheses and artificial sphincters.
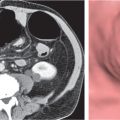
Renal and pancreatic transplants are usually placed within the pelvis (Fig. 29.10). Although they are typically imaged with ultrasound, CT is occasionally performed. The pancreatic transplant is often connected to the bladder by a segment of bowel to allow drainage of exocrine fluids. Care must be taken to avoid confusing this with an abnormal collection. Renal and pancreatic peritransplant collections, such as hematomas, seromas, abscesses, urinomas, lymphoceles, and pancreatic pseudocysts, are readily detected with CT.
The accompanying tables of differential diagnoses of pelvic pathologic processes are predominantly organized by organ of origin. However, because the exact site of origin is not always evident, tables relating to a variety of lesions disseminating within the peritoneal cavity and originating from the pelvic cavity walls are also included. Table 29.1 discusses pelvic GI lesions, Table 29.2 lesions of the female and male pelvis, Table 29.3 urinary bladder lesions, and Table 29.4 peritoneal and extraperitoneal lesions.


















































































Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree