Axial DWI at the level of the centrum semiovale.
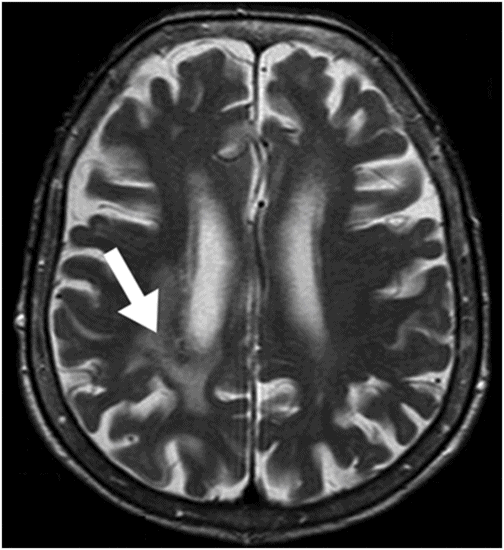
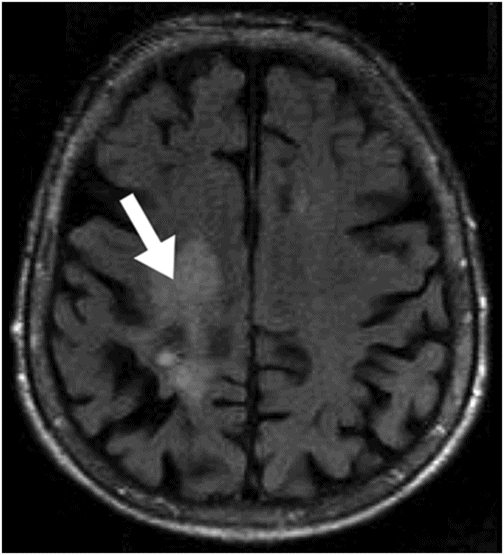
Axial T2WI at the level of the lateral ventricles.
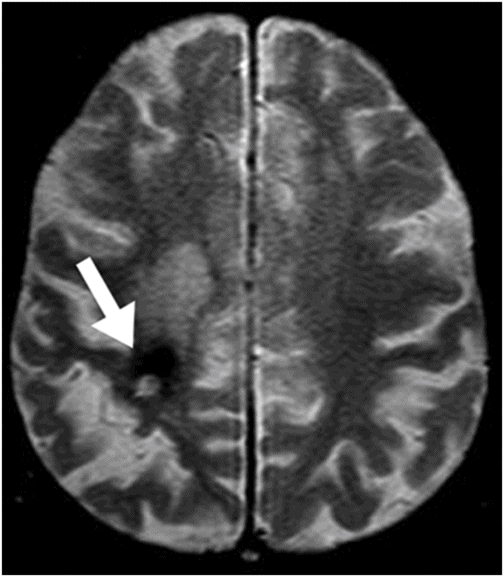
Axial gradient echo (T2*) image at the level of the centrum semiovale.
Developmental Venous Anomaly with Complications
Primary Diagnosis
Developmental venous anomaly with complications
Differential Diagnoses
Developmental venous anomaly associated with a cavernous malformation
Venous thrombosis with venous infarct and hemorrhagic transformation
Imaging Findings
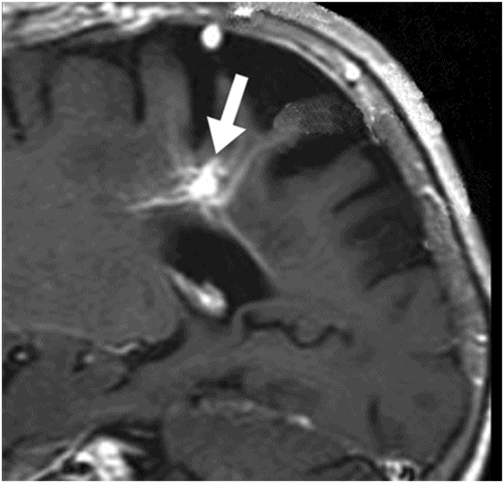
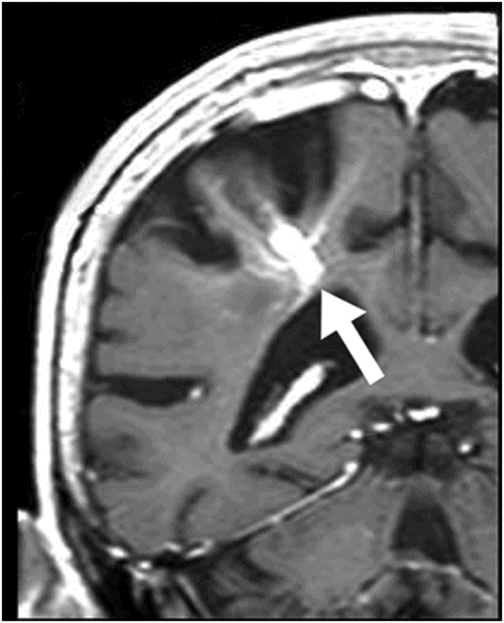
Fig. 31.1: Axial DWI at the level of the centrum semiovale without evidence of an acute ischemic event. Fig. 31.2: Axial T2WI at the level of the lateral ventricles showed ill-defined, subcortical and periventricular hyperintensity in the right parietal lobe with no mass effect (arrow). Fig. 31.3: Axial FLAIR image at the level of the centrum semiovale showed ill-defined, subcortical white matter hyperintensity, with no mass effect, in the right frontal and parietal region (arrow). Fig. 31.4: Axial gradient echo (T2*) image at the level of the centrum semiovale showed an ill-defined, marked subcortical hypointense focus in the right paracentral lobule (arrow). Fig. 31.5: Sagittal and Fig. 31.6: Coronal, zoomed in and enhanced T1WI at the level of the right parietal region demonstrated multiple, small vascular structures converging into a larger vessel (arrow). No other foci on enhancement were seen.
Discussion
The selected images clearly demonstrate a developmental venous anomaly (DVA) draining portions of the right paracentral lobule, with no direct or indirect signs of acute venous thrombosis. There is no evidence of acute hemorrhage or infarct in the adjacent brain parenchyma. The parenchymal volume loss and atrophic changes are most likely due to chronic ischemia, secondary to insufficient venous drainage. The low signal depicted particularly by the gradient echo images can be explained by hemosiderin deposition that is possibly due to chronic petechial hemorrhage.
Cavernous malformations (CMs) are lesions that usually have a popcorn appearance, with mixed and heterogeneous signal on T1- and T2-weighted images and complete hypointense hemosiderin rim. In this patient, there are no signs of an underlying heterogeneous lesion with mixed signal and hemosiderin rim; therefore, this DVA is probably not associated with a CM. The draining vein enhances intensely with contrast and does not demonstrate any increased signal on FLAIR or on GRE sequence (not shown), ruling out isolated cerebral venous thrombosis. Additionally, there are no secondary signs of venous thrombosis.
Venous angiomas, also known as DVA, are considered variations of the normal transmedullary veins that drain the white and gray matter. They are the most frequent vascular malformation DVA, representing 50% of brain vascular anomalies, present in up to 2% of the population. They can be found in any age group, with a slight predominance in males. As opposed to other types of vascular malformation, DVAs have no proliferative potential or arteriovenous shunt with normal interposed brain tissue. It is currently accepted that DVAs act like a compensatory system of venous drainage due to early failure, abnormal development, or an intrauterine occlusion of normal capillaries or small transcerebral veins.
Sixteen percent of patients with DVAs present with multiple lesions. They are found almost anywhere in the brain, more commonly in the frontal and parietal lobes and in the posterior fossa. They are typically unilateral, although bilateral DVAs have been reported, particularly in the posterior fossa. They range from a small venous structure that drains small portions of the brain to large venous anomalies that drain a whole hemisphere. They can coexist in around 13–40% of the patients with cavernous or capillary malformations. Capillary telangiectasias are rarely seen on non-contrast examination. However, they may be depicted as low signal structures on gradient echo images with ill-defined contrast enhancement.
Typically, DVAs are an incidental finding and patients are usually asymptomatic and rarely develop complications. The main complications related to DVAs are venous infarct and hemorrhage. The reported risk of hemorrhage ranges from 0.22% to 0.68% per year. Hemorrhage and venous infarct in a DVA draining territory is usually a result of acute thrombosis of the collecting vein. Thrombosed DVAs may be associated with venous infarct in around one-half of the patients, parenchymal hemorrhage in 37%, intraventricular and subarachnoid hemorrhage in 5%, and no lesions in 5% of the patients. The majority of thrombosed DVAs have favorable outcomes with complete recovery or mild neurologic symptoms.
The most frequently reported abnormalities associated with DVAs are CMs. Other abnormalities include signal abnormalities of the surrounding white matter, calcifications, and brain atrophy.
Cavernous malformations and DVAs are the most frequently mixed vascular malformations, and are more commonly seen in the posterior fossa. Atrophy, with enlargement of sulci and ventricles, and signal abnormalities within the venous drainage territory, can be found in almost one-third of the patients with DVA. Signal changes can be found adjacent to the dilated medullary veins or circumscribe the entire drainage territory of the DVA. These tend to be stable and show no contrast enhancement or restricted diffusion. Dystrophic calcifications can be found in up to 10% of patients with DVA, regardless of their size.
Developmental venous anomalies that form around the mesencephalon or quadrigeminal plate may cause triventricular hydrocephalus if they compress the cerebral aqueduct. In these patients, medical intervention should target the hydrocephalus; however, no treatment is recommended for the DVA. Patients may be treated with shunting or third ventriculostomy.
Non-enhanced CT studies do not usually reveal DVAs. After contrast administration, the venous collector of DVAs is readily detectable as a linear or curvilinear enhancement, travelling from the deep white matter to a cortical or deep vein or to a dural sinus. Small DVAs may be not seen on non-enhanced MRI scans. When visible on MRI, DVAs have a transhemispheric flow void on regular T1- and T2-weighted images. After contrast administration, DVAs are readily depicted because of their usual slow flow. The cluster of small veins with a spoke-wheel appearance drains to a large venous collector, which can extend either to the superficial or deep venous system. This agglomeration of small veins may be called caput medusae (or the head of Medusa). If there is an association between a CM, then susceptibility weighted imaging and conventional T2*-weighted imaging will be most sensitive for this component. Susceptibility weighted imaging is the MRI sequence of choice to demonstrate venous anomalies, with higher sensitivity to depict venous structures than conventional T2*-weighted imaging. Developmental venous anomalies demonstrating parenchymal enhancement may be harbingers of future hemorrhage, indicating venous restrictive disease in association with the draining vein. On digital angiography, DVAs are only seen in the venous phase and no shunt is present. Although DVAs are typically considered inconsequential, it has been shown that the area of brain adjacent to the DVA demonstrates significant hypometabolism in the absence of any structural brain abnormality.
No treatment is necessary in uncomplicated DVAs. If hemorrhage occurs, surgery may be necessary to treat increased intracranial pressure. Patients with CVAs who undergo brain surgery are at an inherent risk of infarct in the event the collecting vein was unintentionally cauterized.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree