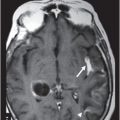
(A–B) Axial T2W images through the level of pons and middle cerebellar peduncles.


HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis
Primary Diagnosis
HTLV-1-associated myelopathy/tropical spastic paraparesis
Differential Diagnoses
Amyotrophic lateral sclerosis (ALS)
Adult-onset Krabbe disease
Adult-onset adrenoleukodystrophy
Toluene abuse
Imaging Findings
Fig. 48.1: (A) Axial T2WI and (B) FLAIR MR images (arrows) revealed nearly symmetric hyperintense signal in the central deep white matter. (C) Axial postgadolinium T1WI did not show enhancement. Fig. 48.2: Axial DWI was unremarkable. Fig. 48.3: (A–B) Axial T2WI demonstrated hyperintense signal abnormality involving the corticospinal tracts and transverse pontine fibers (arrows) and central tegmental tracts (arrowheads). Fig. 48.4: Axial T2WI of the brainstem at the level of medulla showed nearly symmetric hyperintense corticospinal signal abnormality involving the pyramids (arrows).
Discussion
In a patient who is seropositive for HTLV-1 antibodies, who presents with slow progression of spastic paraparesis, cognitive decline, and coexistent bladder dysfunction, a diagnosis of HTLV-1-related tropical spastic paraparesis was considered.
Amyotrophic lateral sclerosis can have similar imaging features but bladder dysfunction is an uncommon association. Similar corticospinal tract involvement and clinical presentation can also be seen in adult-onset adrenoleukodystrophy, adult-onset Krabbe disease, and toluene abuse. However, normal hematologic studies for VLCFAs, galactocerebrosidase enzymatic activity, and negative toxicology analysis helped in excluding these conditions.
HTLV-1 is a retrovirus, causing immune-mediated/direct invasion of the nervous system, which is endemic in well-defined geographic regions such as Japan, the Caribbean islands, South America, the Middle East, and Africa. Approximately 20 million people are infected worldwide and most are asymptomatic carriers. The classical neurologic presentation is called HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). However, HTLV-1 presents with various isolated or assorted syndromes such as myopathy, motor neuron disease, and cognitive decline, ALS-like syndrome, dysautonomia, and polyneuropathy; hence, it should be called HTLV-1-associated neurologic complex.
HTLV-1 neurologic complex has been described as an iceberg model with HAM/TSP forming its tip, which develops in 2–4% of the infected population.
Although the exact mechanism of CNS disease in HTLV-1-associated neurologic complex is unknown, it has been postulated to stem from direct viral invasion and/or autoimmune mechanisms that result in brain and spinal cord infiltration by lymphocytes and monocytes involving the gray and white matter. Patients present with gait and urinary disturbances, constipation, hyperreflexia, and impaired posterior column conduction abnormalities on neurologic examination. The diagnosis is confirmed by detecting anti-HTLV-1 antibodies in CSF and serum.
There are no specific MR imaging features of HTLV infection described in the literature. Multifocal discrete and confluent hyperintense lesions can be found in the subcortical, periventricular, and central deep white matter on T2-weighted and FLAIR images. These imaging findings at times can be confused with demyelinating lesions of multiple sclerosis. Dirty white matter changes and symmetric lesions involving the corticospinal tracts have been described that could mimic ALS on FLAIR and T2W images. Long segment lesions have been described in the cervico-thoracic spinal cord that especially involve the dorsal columns, possibly explaining the posterior column conduction disturbances in these patients. Spinal cord atrophy has been reported in up to 20–70% of cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree