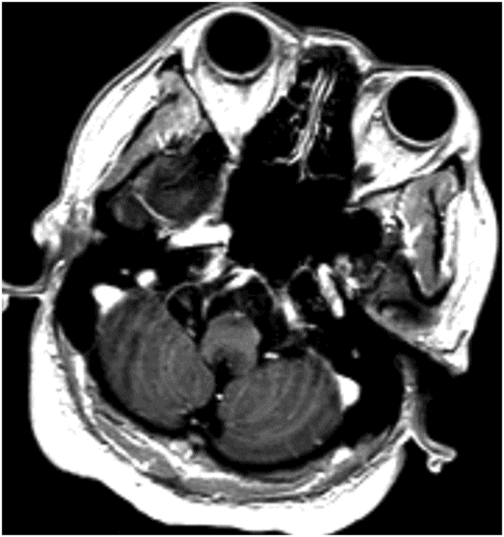
Axial T2WI through the medulla at the level of olive.
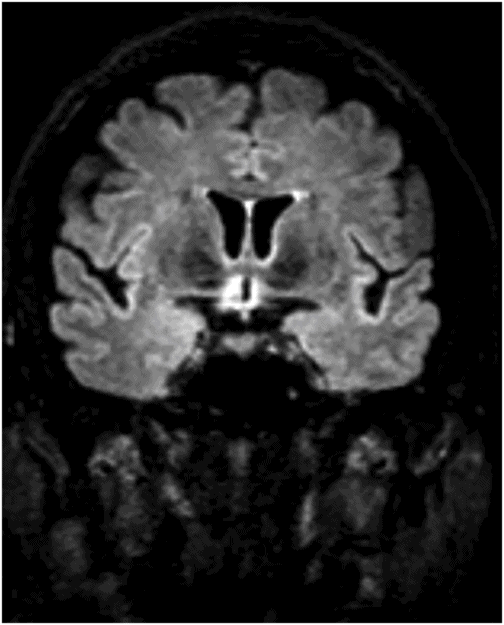
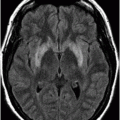
Coronal FLAIR image through the level of the third ventricle/posterior optic chiasm.
Neuromyelitis Optica (NMO) Spectrum Disorder
Primary Diagnosis
Neuromyelitis optica (NMO) spectrum disorder
Differential Diagnoses
Multiple sclerosis
Acute disseminated encephalomyelitis
Paraneoplastic syndrome
Imaging Findings
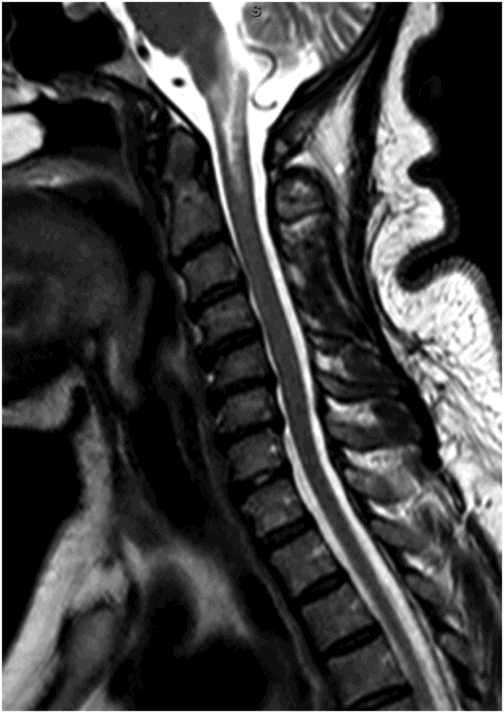
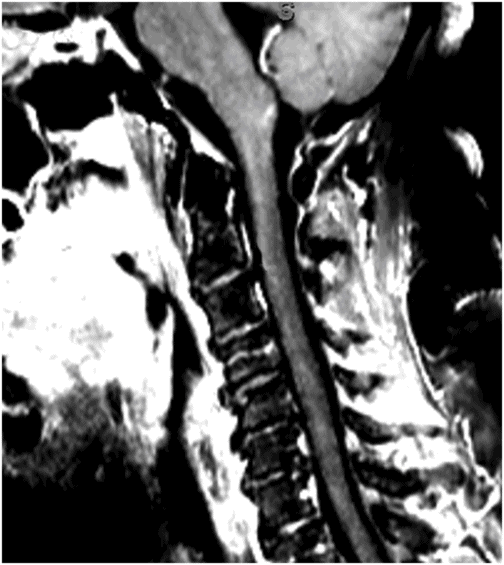
Fig. 59.1: Axial T2WI through the medulla at the level of olive demonstrated T2 hyperintensity surrounding the inferior fourth ventricle in the expected location of area postrema. Fig. 59.2: Axial T1WI with contrast through the medulla at the level of olive demonstrated T1 hypointensity without any enhancement surrounding the inferior fourth ventricle in the expected location of area postrema. Fig. 59.3: Coronal FLAIR image through the level of the third ventricle demonstrated FLAIR hyperintensity involving the periventricular subependymal zone around the third ventricle. Fig. 59.4: Midline sagittal T2WI demonstrated an elongated T2 hyperintense lesion involving the dorsal medulla extending into the superior cervical cord. Fig. 59.5: Midline sagittal T1WI with contrast demonstrated possible subtle enhancement of the dorsal medulla in the area of abnormal T2 hyperintensity.
Discussion
Typical clinical presentation with uncontrolled hiccups and vomiting, typical MRI findings involving the periependymal tissue surrounding the third and fourth ventricles, and positive autoantibodies against aquaporin-4 protein (NMO autoantibody) is diagnostic of neuromyelitis optica spectrum disorder (NMOSD).
Although multiple sclerosis (MS) can present in older adults, the clinical presentation and imaging findings are not typical for MS lesions (see the discussion below). There was no prodromal symptom to suggest acute disseminated encephalomyelitis (ADEM). Simultaneous involvement of hypothalamus and brainstem can be seen in paraneoplastic syndromes with antibodies against Ma-2, which is typically seen in young male patients with germ cell tumors.
Neuromyelitis optica (previously called Devic syndrome) is an autoimmune demyelinating disease previously considered a variant of MS. However, NMO is now classified as a distinct disease entity. Discovery of the correlative relationship between the presence of autoantibodies targeting the aquaporin-4 water channel (AQP4) protein and NMO distinguish it from MS. Aquaporin-4 is a type III transmembrane protein that regulates water entry into and out of specific cells (astrocyte foot processes) in the brain and interfaces with blood vessels within the neuropil and around the ventricles. Although aquaporin-4 regulates water in multiple types of epithelia cell types, it has a critical role when expressed by astrocytes that regulate water and ion movement in parts of the brain.
The serum autoantibody NMO-IgG, which targets aquaporin-4, is more than 90% specific for NMO in patients presenting with an optic-spinal syndrome; however, NMO-IgG is not detected in patients with classic MS. NMO-IgG seropositivity also predicts relapse and conversion to neuromyelitis in patients presenting with a single attack of longitudinally extensive myelitis.
Thus, the most current neuromyelitis optica diagnostic criteria (2006) require optic neuritis, myelitis, and at least two of three supportive criteria: longitudinally extensive transverse myelitis, onset brain MRI that is not diagnostic of MS, or NMO-IgG seropositivity. Additionally, identification of anti-AQP4 antibodies indicates a broader clinical phenotype of this disorder, the so-called NMO spectrum disorder (NMOSD) that includes anti-AQP4 antibody seropositive patients with limited or early forms of NMO presenting with specific brain abnormalities. It also includes patients of systemic lupus erythematosus (SLE) and Sjögren syndrome that are also seropositive for anti-AQP4 antibodies.
This rare inflammatory disease has a severe, relapsing course with a demyelinating component that has a predilection for the optic nerve and spinal cord: it often results in blindness, quadriplegia, and death. Anti-aquaporin-4 antibodies are specific and sensitive for NMO. Anti-aquaporin-4 antibody testing not only identifies individuals who are positive for NMO and present with limited symptoms (neuromyelitis optica spectrum disorder) but also permits identification of seronegative patients. It is important to differentiate patients with NMO from other forms of demyelinating diseases such as MS. Patients with NMO incorrectly diagnosed and treated for MS may experience worsening of symptoms and unwanted side effects if treated with interferon beta and natalizumab.
Imaging plays a key role in the diagnosis of NMOSD. The characteristic imaging findings of NMOSD include diencephalic and dorsal brainstem lesions surrounding the third ventricle, aqueduct, and the fourth ventricle; longitudinally extensive transverse myelitis (≥ 3); and posterior and bilateral optic nerve involvement. Diencephalic lesions surrounding the third ventricle and the aqueduct are frequently asymptomatic but may have hypothalamic symptoms (syndrome of inappropriate antidiuretic hormone secretion, narcolepsy, temperature and satiety imbalance, etc.) in cases of severe involvement. Lesions at the dorsal brainstem surrounding the fourth ventricle in the area postrema and nucleus tractus solitarius are the most specific signs of NMOSD and can be seen in up to 46% of patients. These lesions are associated with intractable vomiting and hiccups, as seen in this patient. Optic nerve lesions tend to involve the posterior aspect of the nerves (prechiasmatic and chiasmatic segments) and are often bilateral. Periependymal lesions that surround the lateral ventricles can be seen in up to 40% of patients, particularly involving the corpus callosum, which can be confused with MS lesions. Acute lesions prefer to involve the inferior surface of the callosum closest to the ventricle, the entire cranio-caudal dimension of the corpus callosum, or in severe cases, they may involve the adjacent cingulum. Hemispheric white matter lesions have also been described in NMOSD that are often tumefactive, have spindle-like appearances and cloud-like enhancement. Both unilateral and bilateral corticospinal tract lesional spread has also been described in NMOSD (up to 44% of patients). Spinal cord lesions preferentially involve the central gray matter area in the cervical and upper thoracic cord. Preferential central involvement is due to more abundant AQP4 expression in the gray matter and in the periependymal tissue surrounding the central canal.
In clinical practice NMOSD needs to be differentiated from MS. Typical peripheral and spinal cord MS lesions demonstrate focal, rather than long segment, involvement, and involve the peripheral brainstem, not central brainstem. Additionally, typical MS lesions have Dawson figure appearance in the periventricular (lateral ventricle) and pericallosal white matter: these areas are usually spared in NMOSD. Enhancement lesions are typically ovoid or open-ring type rather than cloud-like. Additionally, cortical lesions are frequently seen in MS, unlike NMOSD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree