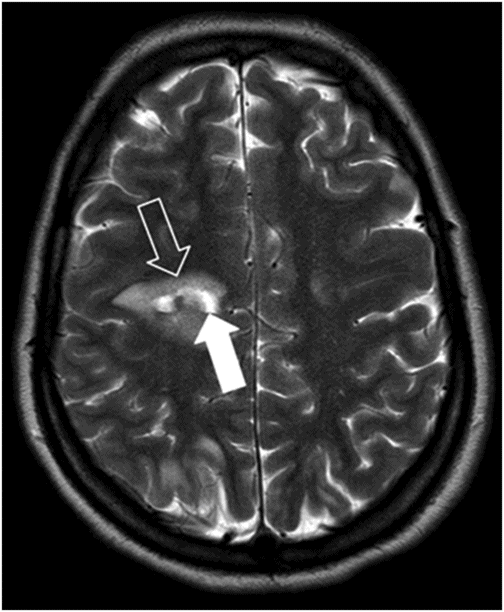
Axial T2-weighted image.
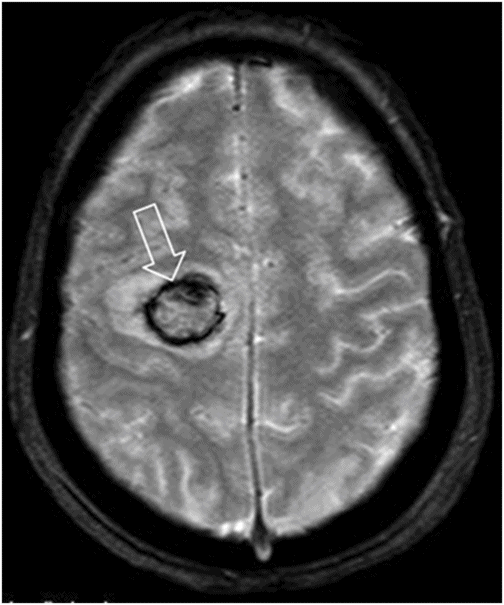
Axial T2* image.
Posterior Reversible Encephalopathy Syndrome with Hemorrhage
Primary Diagnosis
Posterior reversible encephalopathy syndrome with hemorrhage
Differential Diagnoses
Venous sinus thrombosis with venous infarct and hemorrhagic transformation
Reversible cerebral vasoconstriction syndrome
Hypertensive parenchymal hemorrhage
Imaging Findings
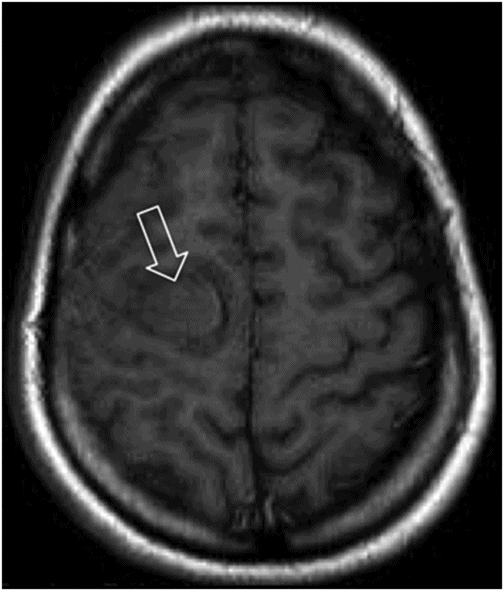
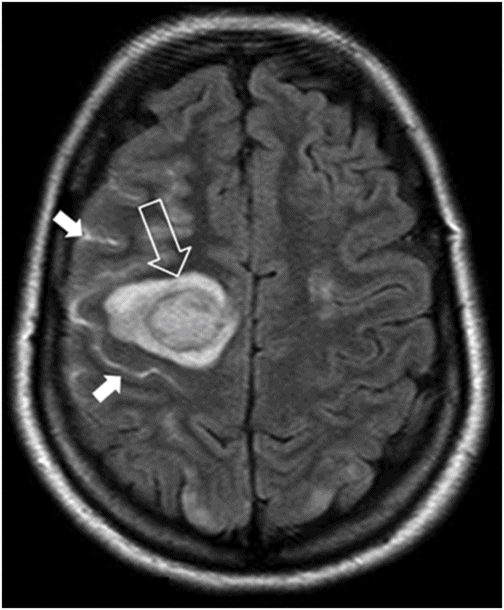
Fig. 63.1: Axial FLAIR image showed multifocal cortical and subcortical hyperintensities (open arrows) representing vasogenic edema with mild mass effect in the posterior aspects in both parietal lobes and in the right frontal region. Fig. 63.2: Axial T1-weighted image demonstrated a subcortical slightly hyperintense focus (open arrow) with nodular appearance and mild mass effect in the right frontal lobe. Fig. 63.3: Axial T2-weighted image demonstrated that the right frontal lobe lesion was associated with fluid-fluid level material (open arrow). The material has hyperintense signal superiorly and low signal in its dependent portion with mild surrounding edema (solid arrow), which is consistent with recent acute hemorrhage. Fig. 63.4: Axial FLAIR image showed a nodular lesion with increased internal signal, more prominent than the brain parenchyma and the CSF. Note that the lesion showed mild perilesional vasogenic edema (open arrow). The increased signal in the subarachnoid spaces of the right frontal gyrus is compatible with hyperproteinaceous material, most likely representing subarachnoid hemorrhage (small solid arrows). Fig. 63.5: Axial T2* image showed a nodular lesion with mixed internal heterogeneous signal with low signal in its periphery (open arrow), consistent with hemosiderin deposition.
Discussion
The findings of bilateral cortical and subcortical vasogenic edema in the posterior portions of the brain parenchyma in a pregnant woman diagnosed with pregnancy-induced hypertension who presented with altered mental status, seizures, and neurologic deficit are highly consistent with posterior reversible encephalopathy syndrome (PRES). The association of PRES with intraparenchymal and subarachnoid hemorrhage is not a typical PRES presentation; however, it has been described as a potential complication of PRES. Approximately 15% of patients with PRES demonstrate hemorrhage. Intracranial hemorrhage can consist of minute focal hemorrhages, sulcal subarachnoid hemorrhage, and focal hematoma, all of which may present in isolation or in combination. Association of PRES with hemorrhage is more typically seen in patients after allogenic marrow transplant and in patients undergoing therapeutic anticoagulation.
Parenchymal changes as described above can be seen as a complication of cerebral venous thrombosis (CVT), particularly if the superior sagittal sinus or cortical veins are involved. Approximately one-third of CVT patients developed intracerebral hemorrhage, which can occur in the brain parenchyma and extra-axial spaces such as the subarachnoid. Owing to local decreased cerebral perfusion, there could be ischemic injury and cytotoxic edema, disruption of the blood-brain barrier leading to vasogenic edema, and venous and capillary rupture culminating in parenchymal hemorrhage. Negative MRI venography findings (not shown) and absence of signal changes in the flow voids make the possibility of venous thrombosis less likely in this patient.
Reversible cerebral vasoconstriction syndrome (RCVS) is a fairly recently described entity that occurs with varied clinical and imaging findings. Patients usually experience a sudden history of severe headache associated with reversible multifocal segmental vasoconstriction of intracranial arteries (vasospasm), and rarely focal neurologic deficit. Reversible cerebral vasoconstriction syndrome can be associated with medication use (sympathomimetic, serotonergic, or other drugs), pregnancy, and puerperium.
Intracranial hemorrhage is a common finding in PRES and is most frequently subarachnoid, occurring secondary to vasospasm. Magnetic resonance images of the brain during the first week appear normal in most patients, with intracerebral hemorrhage occurring in up to 10% of cases. Ten percent of RCVS patients will have MRI abnormalities consistent with PRES, which may have a possible common pathogenetic mechanism with disturbance of vascular tone. Radiographic evidence of infarction, often in the arterial borderzone regions, may be seen during the second week. Magnetic resonance angiography reveals diffuse segmental arterial constriction in up to 90% of cases. In addition, the large- and medium-sized arteries are more commonly affected. The history of headache, pregnancy, and subarachnoid hemorrhage are good reasons to include RCVS in the list of differential diagnoses. The lack of vasoactive agent exposure, a common association with RCVS, the presence of seizures and focal neurologic deficit, uncommon in typical cases of RCVS, and the fact that the MR arterial angiogram was normal (patient could not undergo catheter angiography) make the possibility of RCVS less likely.
Hypertensive intracranial hemorrhage (HIC) is a result of increased hypertension, which causes vessel wall degeneration, atherosclerosis, fibrinoid necrosis, and wall rupture. It is the second most common cause of stroke, which is seen as hyperdense lesions on non-enhanced CT. Hypertensive intracranial hemorrhage is more commonly located in the putamen, external capsule, thalamus, pons, and cerebellum. It manifests as macroscopic hematomas or micro-bleeds. Occasionally, the subarachnoid and the ventricular system can be involved as an extension of the parenchymal hemorrhage. As the majority of the patients with HIC are more than 50 years old and the hemorrhage is lobular (not the usual location), HIC is not likely the definitive diagnosis of this patient.
Posterior reversible encephalopathy syndrome is a clinical and radiologic entity characterized by multiple symptoms such as headache, altered mental status, visual loss, seizures, focal neurologic defects, and loss of consciousness. It typically involves the posterior cerebral vasculature, affecting the parieto-occipital regions, which may be a consequence of reduced sympathetic innervation and impaired vascular autoregulation in this area. Hypertension is the most common cause of PRES, followed by cytotoxic medications (particularly immunosuppressive drugs), preeclampsia or eclampsia, and autoimmune and systemic conditions, including sepsis.
Intracranial hemorrhage is a known complication of PRES, occurring in 5–20% of cases, which can occur secondarily to non-aneurysmal sulcal subarachnoid hemorrhage, to the rupture of pial vessels in the presence of severe hypertension, to impaired cerebral autoregulation, and also secondary to brain reperfusion after ischemic injuries, leading to multifocal brain hemorrhages (hemorrhagic transformation).
Hemorrhage is not common in cases of eclampsia, with the highest rate among patients under immunosuppressive treatment. In immunosuppressed patients, hemorrhage is thought to occur more commonly in patients with a history of allogenic bone marrow transplant, as compared to patients with history of solid organ transplant. Hemorrhage following brain ischemia and infarction is also known to develop in the setting of cerebral vasoconstriction or vasospasm. Hemorrhage in PRES is also more frequent in patients on anticoagulation.
T2-weighted images (TSE and FLAIR) are the MRI sequences of choice to evaluate PRES with hemorrhage. Abnormalities of the subcortical white matter in keeping with vasogenic edema with no enhancement are the rule, but the cortex and the basal ganglia can be eventually affected. Other structures such as the brainstem, cerebellum, and frontal and temporal lobes may also be involved. Perfusion images may play a significant role and can show cerebral hemodynamics related to this condition. As there is hyperperfusion, CBF and CBV are elevated, and time to peak is reduced. Diffusion-weighted positive images (with water molecule motion restriction) indicate that there is cytotoxic edema, pointing towards an unfavorable outcome. Routine T2* gradient echo sequences should always be included in MRI protocols for early detection of blood by-products. If available, more advanced susceptibility-weighted sequences are at least 3–6 times more sensitive than conventional T2*-weighted gradient echo images in detecting subtle minute hemorrhages.
Treatment of the causative factor is typically sufficient to reverse the imaging findings and symptomatology. If prompt diagnosis is not made and treatment is delayed, changes in brain parenchyma (such as brain infarction/hemorrhage) and clinical manifestations may not be reversible. Blood pressure needs to be promptly and cautiously reduced. When immunosuppressive drugs are thought to be the cause, they should be withdrawn quickly to remove the insulting factor to the blood-brain barrier. When hemorrhage is present, surgical treatment is not always necessary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree