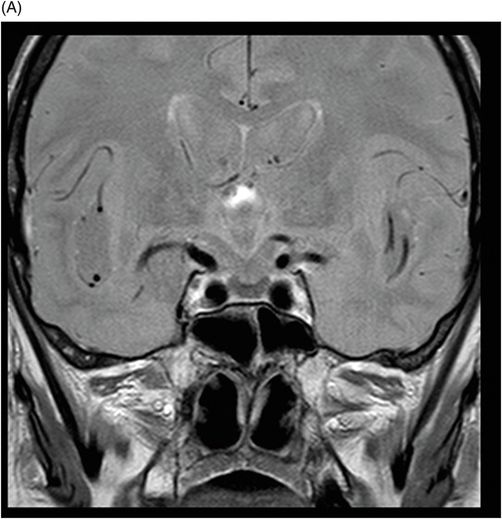
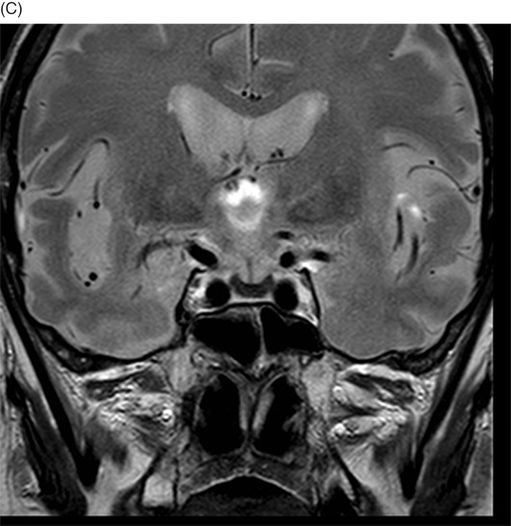
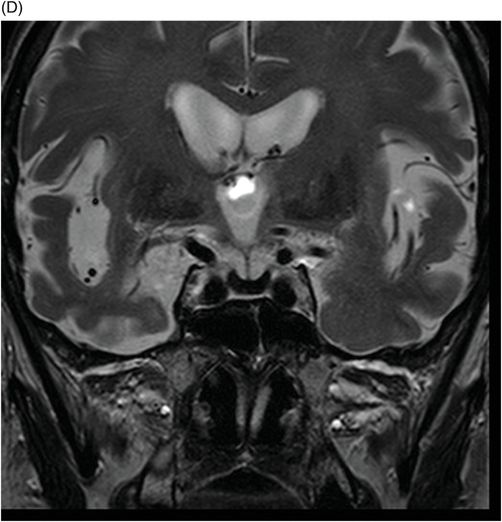
(A–D) Coronal DP and T2WI SE – TE = 60, 80, 120, and 160 ms – through the level of the pituitary gland.
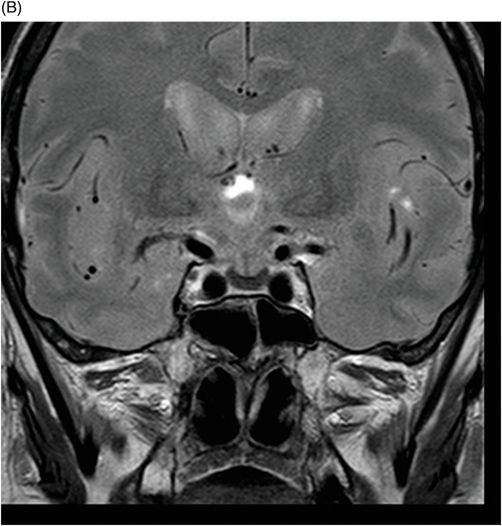
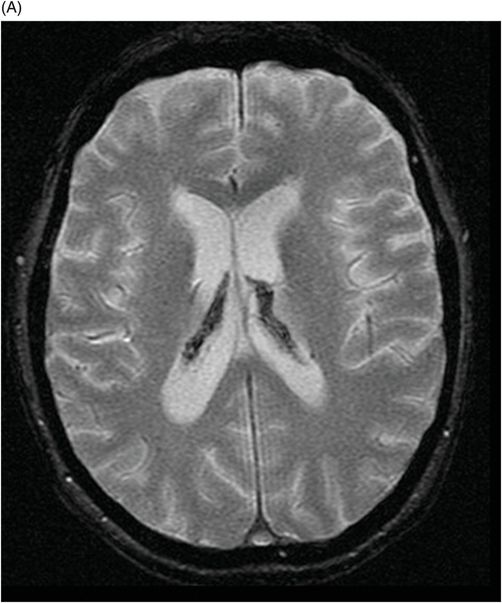
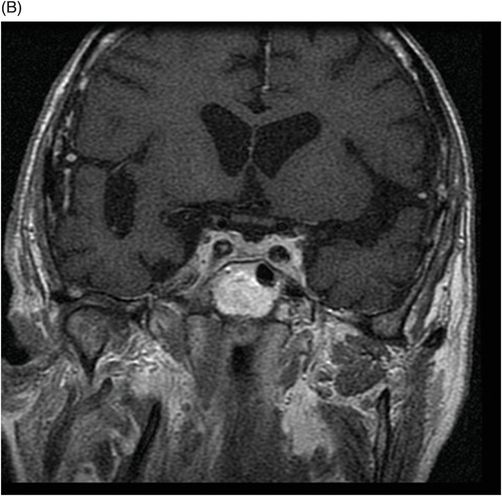
(A) Axial MPGR-T2* and (B) Coronal T1WI FSE postcontrast through the level of the lateral ventricles and pituitary gland.
Hereditary Hemochromatosis
Primary Diagnosis
Hereditary hemochromatosis
Differential Diagnoses
Hemochromatosis
Calcification
Melanoma
Pituitary hemorrhage
Flow voids (aneurysm)
Rathke cleft cysts
Craniopharyngioma
Imaging Findings
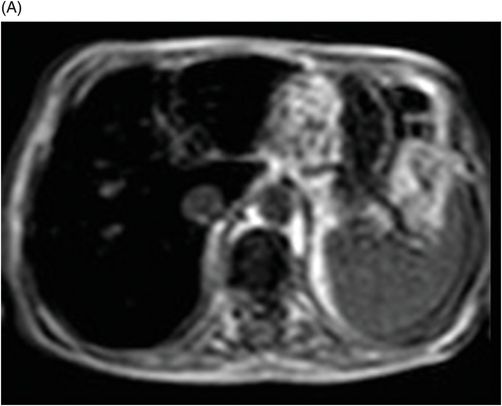
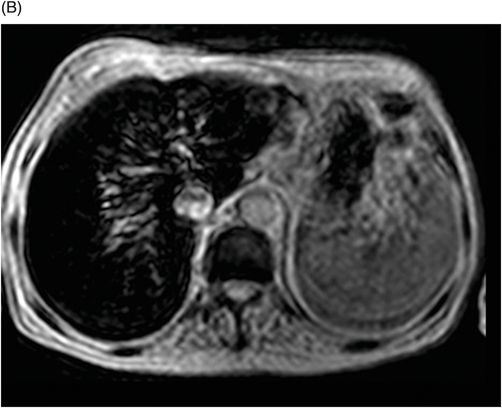
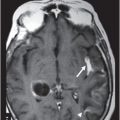
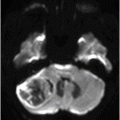
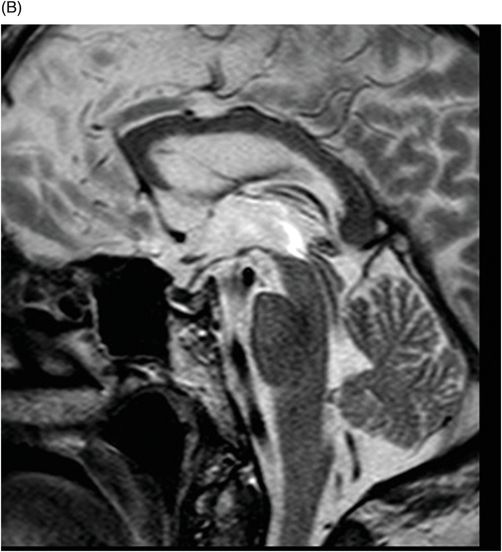
Fig. 76.1 (A) Sagittal T1WI showed a spontaneous hyperintense signal in the topography of the pituitary gland. (B) Sagittal T2WI showed a marked hypointense signal in this topography. Fig. 76.2: (A–D) Coronal DP and T2WI SE, with TE = 60, 80, 120, and 160 ms, demonstrated progressive hypointense signal of the pituitary gland. Fig. 76.3 (A): Axial MPGR-T2* image showed marked hypointense signal in the choroid plexus. (B) Coronal T1WI FSE postgadolinium enhancement of the pituitary gland. Fig. 76.4: (A–B) Upper abdomen MR images showing marked hypointense signal in the liver in the sequences. Axial T1WI in and out of phase.
Discussion
Although hemochromatosis is the only clinical entity associated with both decreased pituitary T2WI signal intensity and serum ferritin values greater than 1000 ng/ml (1000 mg/l), this diagnosis can also be made by exclusion. Other causes of T2 shortening such as primary melanoma are unlikely, because it is exceedingly rare and usually associated with mass effect. An aneurysm is less consistent radiographically, given the lack of signal (abnormal flow void) or structural abnormalities observed in the internal carotid artery. A Rathke cleft cyst or other mass lesion associated with pituitary calcification (craniopharyngioma, chordoma, or pituitary stone) would likely manifest as a focal lesion or a mass effect on adjacent structures, none of which was observed in this patient.
Hemochromatosis is a pathologic state of intracellular iron accumulation in parenchymal tissues. Primary (hereditary) hemochromatosis results from a genetic defect in iron transport, while secondary hemochromatosis results from a variety of acquired etiologies (frequent blood transfusions, chronic hemolytic anemia, or excessive dietary iron intake). Clinical manifestations include fatigue, bronzing of the skin, cirrhosis, cardiomyopathy, diabetes mellitus, and hypogonadism.
Hemochromatosis pathology is based on iron overload resulting from a hereditary/primary cause or from a metabolic/hematologic disorder. Hemochromatosis commonly affects the liver, heart, and endocrine glands. In the brain, iron deposition is typically seen at sites outside the blood-brain barrier, which include the pituitary gland, choroid plexus, pineal gland, and area postrema. Hypogonadotropic hypogonadism can occur early in hereditary hemochromatosis owing to the selective expression of the transferrin receptor by pituitary gonadotrophs, leading to accumulation of intracellular ferritin early in the natural history of the disease.
The reduced signal intensity of the liver on images is caused by the paramagnetic effect of intracellular iron deposits (ferritin and hemosiderin), as occurs in hemochromatosis. This increased iron load results in increased susceptibility. This can be demonstrated on T2 FSE-weighted imaging (Fig. 76.1B), especially in spin echo T2 with progressive increasing echo times (TE) (Fig. 76.2). This feature is more evident on gradient echo or susceptibility-weighted images. The inherent dependence of gradient echo sequences on the effective transverse relaxation time, compared with spin echo sequences, induces a reduction in signal intensity with iron tissue storage, because magnetic field inhomogeneities are not erased with a 180° pulse. In addition, the typical distribution of increased susceptibility involving the following structures, the pituitary gland, choroid plexus, pineal gland, and area postrema, should strongly suggest iron overload.
Upper abdomen MRI (Fig. 76.4 A and B) demonstrated findings consistent with hereditary hemochromatosis. The MR signal from the pancreas may help differentiate between primary and secondary hemochromatosis, because the first usually affects the liver, adrenals, and the pancreas, while the second commonly affects the reticuloendothelial system. However, it is well known that when the amount of iron in the blood system is higher than the capacity that can be stored by the reticuloendothelial system, such as in advanced stages of hemochromatosis or in transfusional siderosis, the pancreas is also involved.
Given the patient’s clinical presentation, laboratory values, and imaging findings, hemosiderin deposition in the anterior pituitary gland secondary to hemochromatosis is the most likely cause of the hypogonadism observed in this patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree