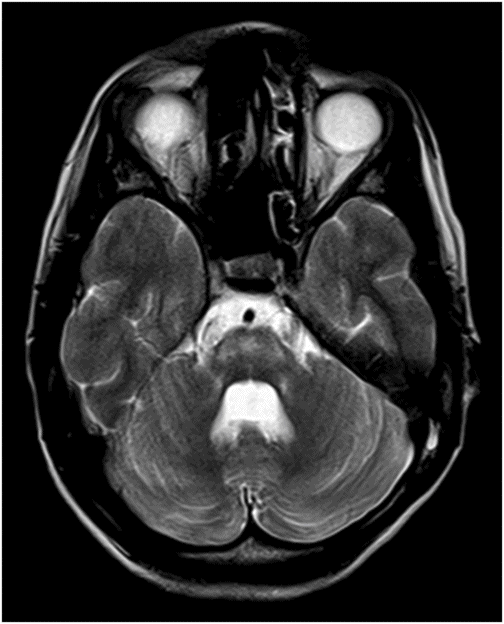
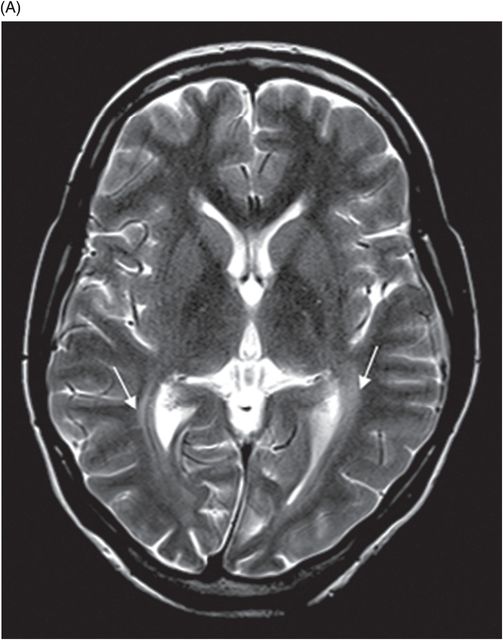
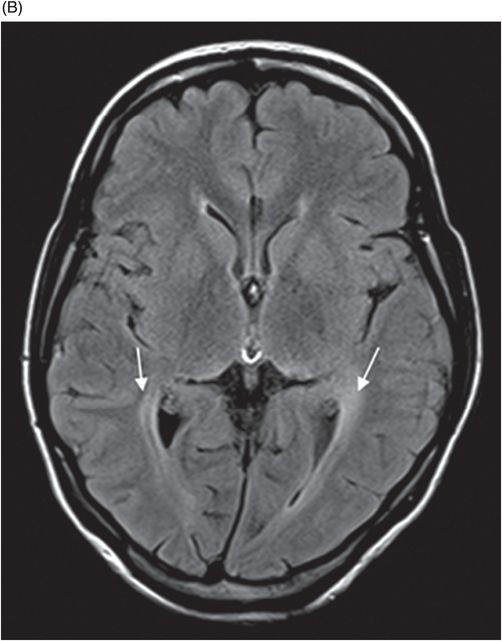
(A) Axial T2WI and (B) Axial FLAIR images at the level of lateral ventricles.
Wolfram Syndrome
Primary Diagnosis
Wolfram syndrome
Differential Diagnoses
Multiple system atrophy
Spinocerebellar ataxia type 3 (Machado-Joseph disease)
Dentatorubral pallidoluysian atrophy
Friedrich ataxia
Leber hereditary optic atrophy
Imaging Findings
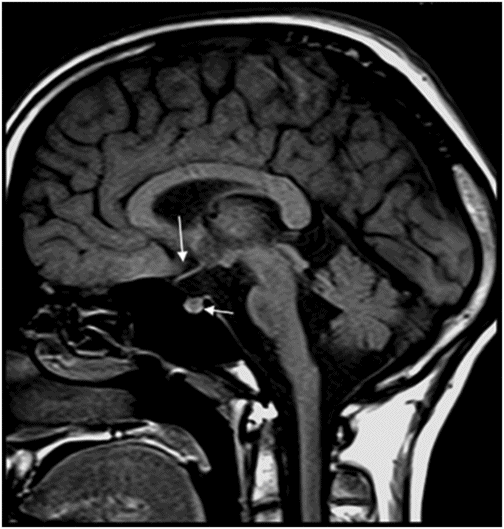
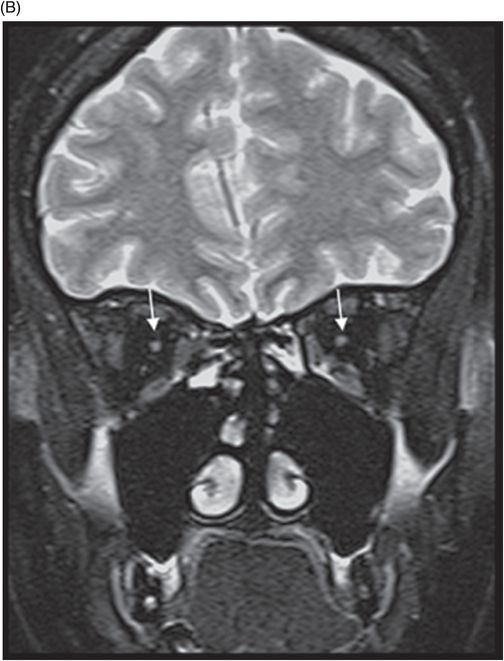
Fig. 77.1: (A) Coronal T1WI image of the orbits showed diffuse thinning of the bilateral optic nerves (black arrows). (B) Corresponding coronal fat-saturated T2WI demonstrated hyperintense signal (white arrows). Fig. 77.2: Sagittal T1WI image shows absence of posterior pituitary bright signal (small arrow) and thinning of optic chiasm (arrow); also noted is brainstem and cerebellar atrophy. Fig. 77.3: (A) Axial T2WI of the brain at the level of lateral ventricles demonstrated symmetric, high signal changes in the bilateral optic radiations (arrows), especially in the peritrigonal white matter. (B) Axial FLAIR demonstrated similar findings. Fig. 77.4: T2WI of the brainstem demonstrated cerebellar and pontine atrophy with T2 hyperintense signal changes in bilateral middle cerebellar peduncles and pons.
Discussion
A clinical presentation of diabetes insipidus, diabetes mellitus, optic pallor (suggestive of optic atrophy), and deafness is highly suggestive of Wolfram syndrome (WS). Neuroimaging findings comprising optic atrophy, brainstem and cerebellar atrophy, and absent normal T1 hyperintense signal of the posterior pituitary gland with endocrinology correlation confirm the diagnosis of WS.
The brainstem imaging features share a considerable overlap with spinocerebellar ataxia type 3, dentatorubral pallidoluysian atrophy, and Friedrich ataxia. However, these neurodegenerative conditions have a predominant brainstem and cerebellar volume loss, with involvement of the superior cerebellar peduncle. The cruciform hyperintense signal on T2WI involving the middle cerebellar peduncles closely resembles multiple system atrophy, but differs in the age of presentation. Patients with Friedrich ataxia also have diabetes mellitus and visual manifestations; however, cerebellar and brainstem symptoms predominate over other signs. In addition, spinal cord involvement is another differentiating feature. Leber hereditary optic atrophy, a distinct hereditary optic neuropathy with mitochondrial inheritance, needs to be considered. It has predilection for the visual pathway and the spinal cord and is often misdiagnosed as multiple sclerosis. The preserved normal high T1 signal in the posterior pituitary and lack of endocrinology and auditory symptoms are important findings.
Wolfram syndrome is a rare genetic disorder first described by Wolfram and Wagener in 1938. It consists of juvenile-onset non-autoimmune diabetes insipidus, diabetes mellitus, optic atrophy, and deafness, and is referred to as DIDMOAD (diabetes insipidus, diabetes mellitus, optic atrophy, and deafness). With an autosomal recessive or mitochondrial pattern of inheritance, the prevalence of WS ranges from 1:100,000 to 1:700,000 and is caused by a WFS1 gene mutation located on chromosome 4p16.1 that encodes the wolframin protein.
Wolfram syndrome has a typical clinical presentation consisting of progressive diabetes insipidus, optic atrophy, deafness, urinary tract obstruction, and large atonic bladder with sphincter dyssynergia incontinence. Neuropsychiatric symptoms have also been described with WS in approximately 60% of patients. Diabetes mellitus is often the first disease symptom, usually diagnosed very early in childhood and followed by optic atrophy, around 10–11 years of age.
On imaging, brain CT is usually normal but cerebellar and brainstem atrophy changes have been reported. However, MR imaging is more specific and sensitive, with diffuse atrophy of the optic nerves and chiasm on T1 and T2 coronal images. The abnormal high T2 signal in the periventricular locations involving the optic radiation is secondary to significant axonal loss with demyelination and gliosis. Diabetes insipidus is attributable to hypothalamic degeneration with loss of vasopressin-secreting neurons in the supraoptic and paraventricular nuclei resulting in loss of normal high T1 signal in the neurohypophysis. Hypothalamic degeneration and loss of pancreatic islet cells has been hypothesized as the cause of diabetes mellitus. Degeneration of pontine and vestibulocochlear nuclei and inferior colliculi, and ballooning of Purkinje cells has been found on autopsy studies and explains high T2 signal involving the pontocerebellar tracts and cerebellar atrophy, as well as the neurologic symptoms.
The imaging features of WS overlap with several neurodegenerative pathologies and hereditary optic neuropathies. Early diagnosis and treatment with hormonal therapy can improve the quality of life. The presence of optic atrophy and signal changes in the visual pathway, loss of normal high T1 signal in the neurohypophysis, and brainstem and cerebellar volume loss, combined with non-autoimmune juvenile diabetes mellitus should help in clinching the diagnosis of this rare neurodegenerative disorder.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree