Sagittal T1WI.
Axial FLAIR image.
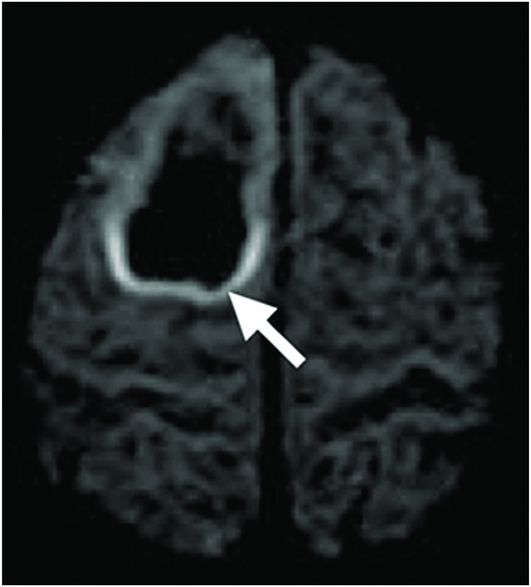
Axial DWI.
Protoplasmic Astrocytoma
Primary Diagnosis
Protoplasmic astrocytoma
Differential Diagnoses
Dysembryoplastic neuroepithelial tumors
Fibrillary astrocytoma
Glioblastoma
Imaging Findings
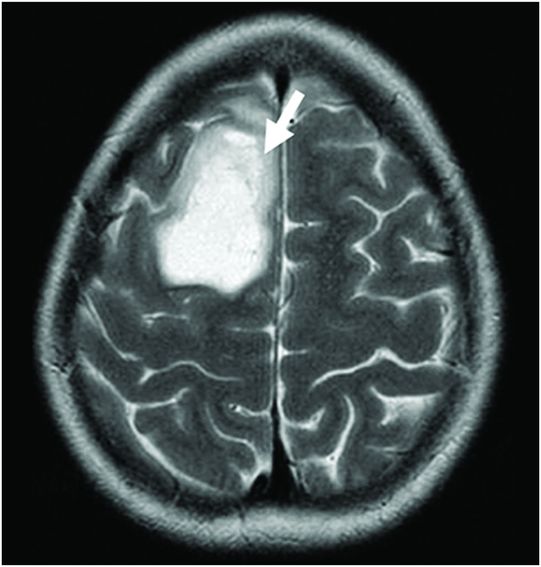
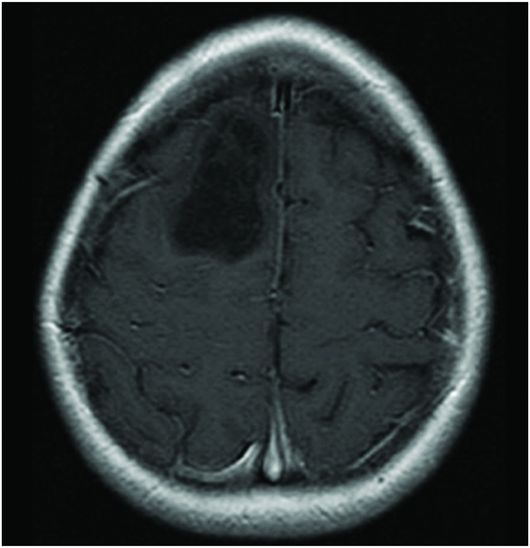
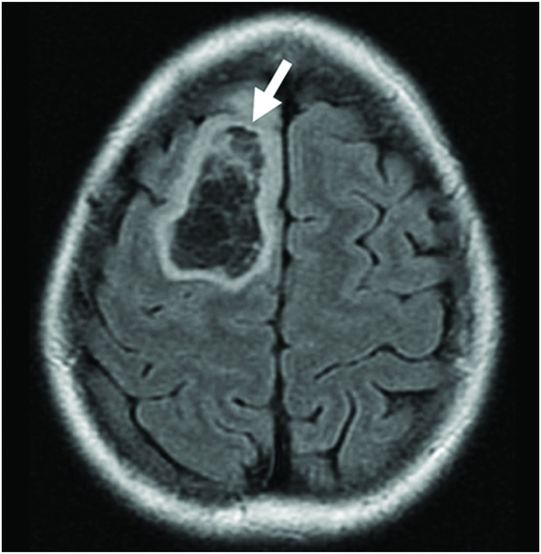
Fig. 86.1: Sagittal T1WI showed a hypointense mass in the right frontal lobe, mainly subcortical in location (arrow). Fig. 86.2: Axial T2WI demonstrated that the lesion had marked, increased homogeneous signal with expansion of the right superior frontal gyrus. The mass involved the subcortical white matter and adjacent cortex (arrow), with mild mass effect but no significant adjacent vasogenic edema. Fig. 86.3: Axial FLAIR image showed marked T2 signal attenuation inside the mass, which also showed internal stranding (septations) and thick ring of peripheral hyperintensity, lacking significant edema (arrow). Gradient echo images did not demonstrate any signs of gross calcification or hemorrhage inside the mass (images not shown). Fig. 86.4: Axial enhanced T1WI showed no significant enhancement inside or in the periphery of the lesion. Fig. 86.5: Axial DWI showed an incomplete ring of increased signal (arrow), with mildly decreased ADC values (images not shown).
Discussion
The imaging findings described above are quite typical for a protoplasmic astrocytoma (PA).
Typically, PAs are masses that demonstrate marked T1 hypointense (Fig. 86.1) and T2 hyperintense (Fig. 86.2) signal with no significant mass effect. These masses have internal FLAIR signal suppression with a variable ring that lacks suppression (increased signal) surrounding the mass (Fig. 86.3). Protoplasmic astrocytomas typically demonstrate minimum or no contrast enhancement. An important feature of PAs is the presence of a complete/incomplete ring of increased diffusion weighted imaging signal (Fig. 86.5).
Based on the imaging features, this lesion could be confused with a dysembryoplastic neuroepithelial tumor (DNET), in which suppression of internal FLAIR signal has been also described (see Part VI: Case 111). However, DNETs tend to be smaller than PAs, with a mean diameter of 30 mm compared to a diameter of around 54 mm for PAs. Similarly, DNETs also involve the cortex, whereas subcortical white matter is more commonly involved in cases of PAs. Another distinct feature of DNETs is that they tend to be triangular in shape, tapering towards the ventricle. Both DNETs and PAs may show portions with marked T2-FLAIR signal suppression (FLAIR hypointense signals); however, PAs exhibit more extension. The peripheral, FLAIR hyperintense rim tends to be thicker and more pronounced in cases of PAs than in DNETs.
Fibrillary astrocytomas (FAs) share imaging characteristics with PAs, as they both demonstrate iso- to hypodensity with no contrast enhancement on CT. On MRI, they can also demonstrate T1 hypointense and T2 hyperintense signal with little or no significant enhancement. The absence of internal FLAIR suppressed/hypointense signal, however, is what differentiates FAs from PAs.
The marked T2 hyperintensity and FLAIR signal suppression/hypointensity may resemble cystic lesions or even necrotic tumors such as glioblastoma. Glioblastoma is the least likely differential diagnosis as these tumors are highly malignant, and typically show necrotic components, contrast enhancement, hemorrhage, neovascularity, high CBV values, and high lactate peaks.
Protoplasmic astrocytoma is a rare variant of low-grade astrocytomas (LGAs), comprising 5% of LGAs, with fairly characteristic imaging and histology features. The age of presentation ranges from 2.5 to 41 years of age (mean, 21 years), with a sex predilection for males. The majority of patients present with seizures and, less commonly, headaches. Hydrocephalus, focal neurologic deficit, and even personality changes can be found, depending on the lesion size and location.
Symptoms can last from 7 months to 28 years (mean duration, 6.6 years). Few patients succumb to the disease.
Classified as WHO grade II tumors, PAs are composed of neoplastic astrocytes with rounded prominent nuclear contour and little cytoplasm. The PA tumoral matrix consists of numerous, prominent microcystic spaces filled with mucinous fluid. Mitoses, microvascular proliferation, and necrosis are absent. Protoplasmic astrocytomas tend to have indolent behavior and occur in younger-aged patients. These tumors have a tendency to be large, well defined, and more commonly located in the cortical and subcortical white matter of the temporal and frontal lobes.
On CT, PAs appear as hypodense masses with variable mass effect, typically with no significant enhancement. On MRI, the tumoral areas show hypointense signal, compared to white matter, on T1-weighted images. On T2-weighted images, PAs show marked and intense hyperintense signal, without vasogenic edema, hemorrhage, or calcification. One important feature of PAs is FLAIR suppressed signal of T2 hyperintense portions of the tumor, indicating that portions of the tumor are cystic in nature. The areas of FLAIR signal suppression are varied, representing more than 50% of the global tumoral extent. Areas of FLAIR hypointensity should not be mistaken for cystic degeneration of necrotic areas, commonly seen in higher grades of glioma.
Typically, PAs do not enhance or demonstrate mild enhancement. On perfusion studies, these lesions usually show very low CBV in relation to normal white matter. On spectroscopy, PAs typically show high choline peaks, higher choline-to-creatine ratio, attenuation of NAA peaks, and markedly high choline-to-NAA ratios, indicating a high-grade lesion. It is important to note that spectral analysis of the cystic components of PAs do not demonstrate elevated lipid/lactate peaks nor show depletion of other metabolites, which are typically seen in true necrotic lesions.
Despite treatment, PAs have a similar outcome to other LGAs, although they apparently have a better prognosis than other subtypes of LGAs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree