DRUG-INDUCED LUNG DISEASE
Drug-induced lung injury is an underreported cause of acute and chronic lung disease.1.2.3.4.5.6.7.8.9.10.11.12.13.14.15.16.17.18.19.20.21.22.23.24. and 25. It affects up to 10% of patients receiving chemotherapy for hematologic and solid organ malignancies.26.27.28. and 29. Lung injury caused by other drugs occurs less frequently and is often less predictable. 11
The precise mechanisms by which drugs cause lung injury are complex and beyond the scope of this discussion;9.27.30. and 31. the interested reader is directed to an in-depth review. 9 In general, there are many potential pathways to drug-induced lung injury. These include (but are not limited to) direct toxic action on lung tissue either by the drug, or, more likely, by products of its metabolism or bioactivation (including toxic oxygen species and free radicals); by a hypersensitivity response to either the drug or its metabolites; by neurally or humorally mediated alterations in capillary permeability; or by induction of an autoimmune state. Further, multiple mechanisms may operate in a given patient treated with a given drug; and many drugs have multiple mechanisms of action. 9
Although use of pharmacologic agents for a variety of conditions is widespread, only a minority of treated patients develops lung toxicity. Causes for individual susceptibility include: the condition treated; occupational factors; associated treatments (concomitant drugs, radiation, or oxygen therapy); renal or hepatic failure; rate of drug infusion and total dose; genetic predisposition; or concomitant administration of drugs that affect metabolism (e.g. the cytochrome P450 pathway). 32
Diagnosis of drug-induced lung disease is difficult because the clinical manifestations are often nonspecific and may be attributed to infection, radiation pneumonitis, or recurrence of the underlying disease. Further, use of combination drug regimens may not only increase risk of drug-induced lung disease, but can greatly complicate diagnosis. Reduction in diffusing capacity for carbon monoxide (DLco) is an important and early clinical finding of many forms of drug-induced lung injury. 33 Early recognition is important because undiagnosed injury can be progressive and fatal, whereas cessation of therapy can result in stabilization or even reversal of disease.
The radiographic, imaging, and histopathologic manifestations of drug-induced lung disease are, with the possible exception of amiodarone toxicity, 34 relatively nonspecific. Prompt diagnosis of drug-induced lung disease requires, first and foremost, a high index of clinical suspicion. Diagnosis also requires careful correlation of clinical symptoms, history of drug exposure, reduction in DLco, appropriate radiographic or imaging findings, exclusion of other causes of injury, and response to therapy (see Box 9.1). Many, if not most, cases are diagnosed clinically, without histopathologic confirmation. The histopathologic findings of drug-induced lung disease (see Table 9.1) reflect a variety of patterns that, while stereotypical, are often non-specific. Lung biopsy specimens may be obtained, however, not necessarily to diagnose drug toxicity, but to exclude other causes of lung injury prior to the institution of therapy. The techniques of treatment are withdrawal of the offending agent, supportive care, and, in some instances, administration of corticosteroids.
Box 9.1
• Appropriate history of drug exposure
• Dose
• Duration
• Concomitant drug, radiation, oxygen therapy
• Clinical signs and symptoms
• Dyspnea
• Cough
• Fever
• Decreased gas transfer
• Compatible radiographic and imaging findings
• Exclusion of alternative diagnoses
• Infection
• Malignancy
• Preexisting lung disease
• Response to therapy
• Withdrawal of offending agent
• Corticosteroids
• Histopathology (rarely obtained)
• Stereotypical, but nonspecific in most cases
• Primarily used to exclude other diagnoses
• Uncommonly pursued except in difficult cases
| BCG, bacille Calmette Guérin. | |
| *Note, this list is incomplete. For more complete list of drugs see Foucher and Camus8and Flieder and Travis. 10 | |
| Histopathologic pattern | Drugs |
|---|---|
| Diffuse alveolar damage | Amiodarone, bleomycin, busulfan, carmustine (BCNU), cyclophosphamide, methotrexate, mitomycin, melphalan, gold salts |
| Interstitial pneumonia | Amiodarone, methotrexate, carmustine (BCNU), chlorambucil, cyclophosphamide, nitrofurantoin |
| Organizing pneumonia | Bleomycin, gold salts, methotrexate, amiodarone, nitrofurantoin, penicillamine, sulfasalazine, cyclophosphamide |
| Eosinophilic pneumonia | Penicillamine, sulfasalazine, nitrofurantoin, nonsteroidal anti-inflammatory drugs, para-aminosalicylic acid |
| Alveolar hemorrhage | Anticoagulants, amphotericin B, cytosine arabinoside (Ara-c), penicillamine, cyclophosphamide |
| Pulmonary vasculitis | Mitomycin, penicillamine, leukotriene antagonists, drug-abuser’s lung |
| Pulmonary edema | Aspirin, cocaine, heroin, morphine, magnesium sulfate, mitomycin, terbutaline |
| Pulmonary hypertension | Fenfluramine and phentermine (Fen-Phen), aminorex |
| Granulomatous inflammation | Methotrexate, nitrofurantoin, sirolimus, interferons, intravesical BCG |
Chest radiography is the primary imaging technique for patients with suspected drug-induced lung disease. Computed tomography (CT) can also be useful in this regard.35.36.37.38.39. and 40. CT, particularly high-resolution CT (HRCT), can detect findings of drug-induced lung disease at an earlier stage than chest radiography. CT may also better categorize the type of pulmonary reaction. Padley et al., 37 for example, were able to distinguish drug-induced cases of diffuse alveolar damage from hypersensitivity reactions using CT. Amiodarone toxicity, however, may be one of the conditions in which CT can be more definitive by virtue of the high attenuation values of amiodarone deposits in the lung.41.42.43. and 44.
Although the radiographic and imaging features of drug-induced lung injury are nonspecific, they often reflect the underlying histopathologic pattern of lung injury. These patterns are fairly characteristic and are often associated with specific drugs. 26 Thus, knowledge of these patterns and of the drugs most frequently involved can facilitate diagnosis of drug-induced lung disease. Table 9.1 and Box 9.2 summarize some of the more important histopathologic and clinicopathologic manifestations of drug-induced lung injury. Unfortunately, many drugs can cause multiple clinical and histopathologic patterns of toxicity, greatly increasing difficulty in diagnosis. Again, a high index of suspicion is required.
Box 9.2
• Injury due to toxic action on lung tissue
• Diffuse alveolar damage
• Interstitial pneumonia
• Organizing pneumonia
• Injury due to hypersensitivity reactions
• Drug-induced asthma
• Eosinophilic pneumonia
• Injury due to neural or humoral mechanisms
• Drug-induced pulmonary edema
• Drug-induced asthma
• Injury due to autoimmune mechanisms
• Drug-induced lupus
• Drug-induced vasculitis
• Drug-induced pulmonary granulomatosis
• Granulomatous vasculitis
• Drug-induced sarcoidosis
• Exogenous lipoid pneumonia
• Miscellaneous
• Drug-induced pulmonary hemorrhage
• Drug-induced vasospasm
• Drug-induced pulmonary thromboembolism
• Drug-induced pulmonary calcification
• Drug-related pleural effusions and fibrosis
• Drug-related lymphadenopathy
• Drug-induced infection
The most important clinical, histopathologic, and radiographic and imaging manifestations of drug-induced lung injury are reviewed first and then selected drugs are discussed. It should be noted, however, that almost 400 drugs are now reported to cause pulmonary toxicity, and the number and range of reported complications increases almost daily. 32 The interested reader is referred to a very comprehensive, and constantly updated, online database of drugs and their known pulmonary complications (www.pneumotox.com). 8
LUNG INJURY DUE TO DIRECT TOXIC ACTION ON LUNG TISSUE
Few drugs are directly toxic to lung tissue. Rather, most exert adverse effects through bioactivation, conversion to various metabolites, or release of toxic oxygen species and free radicals.9.27.30. and 31. The production of excessive reactive oxygen metabolites and a disturbance of the complex oxidant–antioxidant system may result in damage to cells and cell membranes. Alveolar macrophages, polymorphonuclear leukocytes, and lymphocytes may be damaged, resulting in the release of leukokines, lymphokines, and other humoral factors with chemotactic, cytotoxic, or immunomodulating effects. The balance between collagen production and collagen lysis may be altered, causing excess collagen deposition and thus leading to pulmonary fibrosis. The collagen itself may be disordered either by damage to the fibroblasts or by the action of anticollagen antibodies. Inflammatory cells release proteolytic enzymes, and a complex antiprotease system exists to combat the effects of these enzymes. Inhibition of the antiprotease systems can also result in toxicity.
Toxic action on lung tissue usually takes some time to reach a clinically appreciable threshold, and thus the effects may not be apparent for weeks or months after treatment has begun. In many cases the effect is dose related, a relationship that is most evident with the cytotoxic agents bleomycin, busulfan, and carmustine (BCNU). Lung toxicity may be accentuated by other factors such as increasing age, decreased renal function, radiation therapy, oxygen therapy, and other cytotoxic drug therapy. 27 Toxic action of drugs on the lung manifests histopathologically with at least three important patterns of lung injury: diffuse alveolar damage, interstitial pneumonia, and organizing pneumonia.
Diffuse alveolar damage
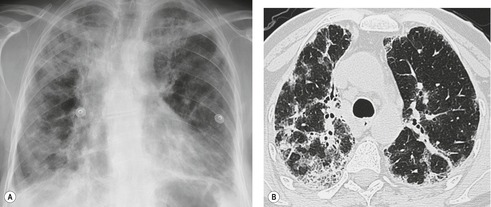
Diffuse alveolar damage is a descriptive term for the sequence of events that occurs after acute severe lung injury that results in necrosis of type I pneumocytes and alveolar endothelial cells. 26 Drugs that most frequently cause diffuse alveolar damage include bleomycin, busulfan, carmustine (BCNU), cyclophosphamide, etoposide, gefitinib, gemcitabine, irinotecan, melphalan, methotrexate, mitomycin, and gold salts (see Table 9.1).10.16.25.34. and 45. At least two histopathologic phases of diffuse alveolar damage are recognized: an acute exudative phase and an organizing reparative phase.45. and 46. The exudative phase occurs in the first week after lung injury and is characterized by edema and intraalveolar membranes composed of necrotic epithelial cells and protein-rich fluid.26.45. and 46. The reparative phase typically occurs after 1–2 weeks and is characterized by proliferation of type II pneumocytes and fibrosis.26.45.46. and 47. Depending on the severity of the injury, diffuse alveolar damage can progress to end-stage fibrosis. Diffuse alveolar damage is, however, sometimes reversible and many survivors have minimal residual functional abnormalities. 26
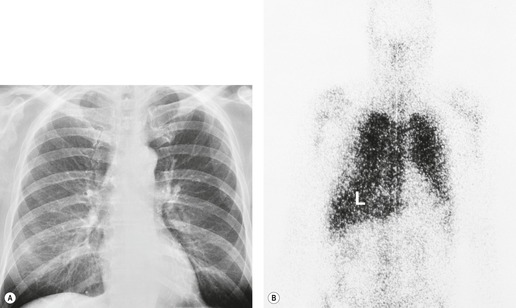
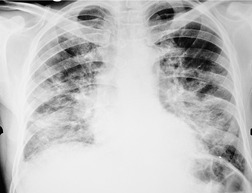
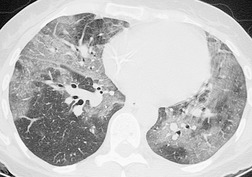
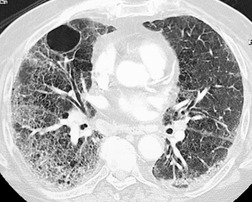
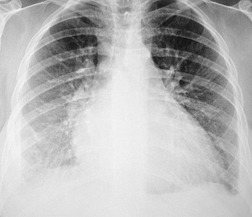
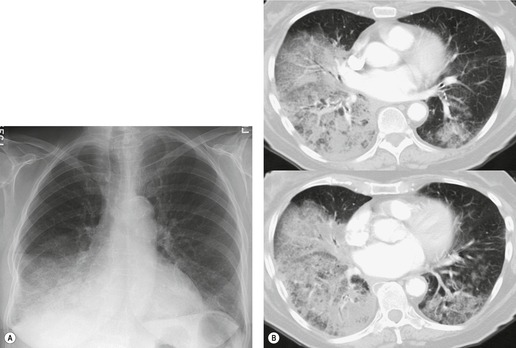
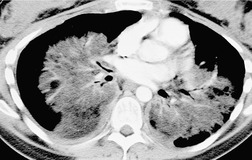
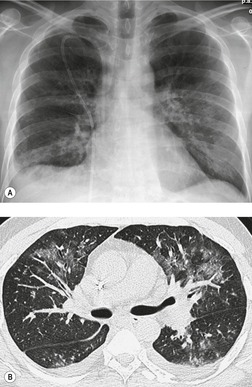
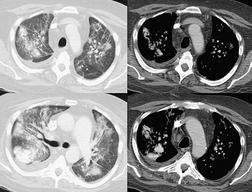
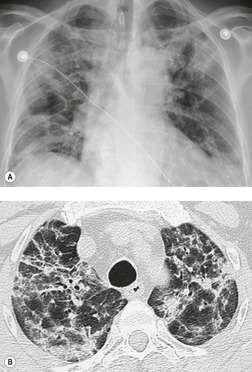
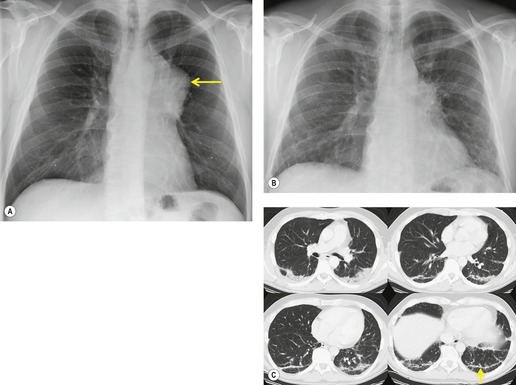
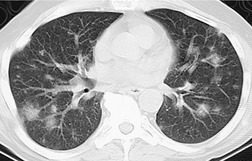
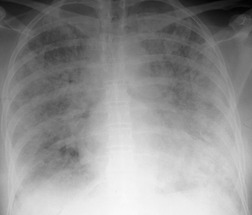
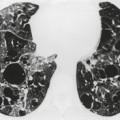
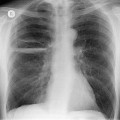
The chest radiograph is often normal in early drug-induced diffuse alveolar damage. In this setting, however, gallium-67 scan or CT, pulmonary function tests, or lung biopsies can show direct or indirect evidence of lung toxicity (Figs 9.1 and 9.2). As disease progresses, however, chest radiographs show bilateral, scattered or diffuse heterogeneous or homogeneous opacities (Fig. 9.3). The opacities may be more apparent in the lung bases. Segmental or lobar consolidation is not a typical feature and pleural effusion is rare. HRCT can be quite useful in early drug-induced diffuse alveolar damage by showing scattered or diffuse ground-glass opacities (Fig. 9.4), often when the chest radiograph is normal (Fig. 9.2) or equivocal. HRCT performed in the late, reparative phase of diffuse alveolar damage may show findings of fibrosis such as irregular linear opacities, architectural distortion, and traction bronchiectasis (Fig. 9.5).
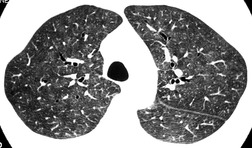 |
| Fig. 9.2 (Reprinted from Rossi SE, Erasmus JJ, McAdams HP, et al. Pulmonary drug toxicity: radiologic and pathologic manifestations. RadioGraphics 2000;20:1245–1259. Copyright Radiological Society of North America.) |
As drug-induced diffuse alveolar damage mostly occurs in patients with malignancy, the differential diagnosis includes pulmonary edema, lymphangitis carcinomatosa, leukemic or lymphomatous infiltration of the lung, opportunistic infections such as Pneumocystis jirovecii pneumonia, and pulmonary hemorrhage (see Chapter 6). The clinical circumstances and sequence of events may suggest drug-induced diffuse alveolar damage as the cause. Lung biopsy may, however, be necessary to exclude other conditions.
Interstitial pneumonia
Interstitial pneumonia is probably the most common histopathologic manifestation of drug-induced lung disease. 10 A variety of patterns are reported, including a usual interstitial pneumonia (UIP)-like pattern, lymphoid interstitial pneumonia (LIP) and nonspecific interstitial pneumonia (NSIP). Of these, the NSIP pattern is the most common. NSIP is a term used to describe interstitial inflammation and fibrosis that does not fulfill diagnostic criteria for usual, desquamative, or acute interstitial pneumonia. NSIP is characterized histopathologically by scattered infiltrates of mononuclear inflammatory cells, mild interstitial fibrosis, and reactive hyperplastic type II pneumocytes. 45 However, use of the term NSIP in the setting of drug-induced lung disease is controversial. Some authors prefer to reserve the term NSIP for cases of idiopathic disease and to use the term cellular interstitial pneumonia (CIP) or just interstitial pneumonia for cases of drug-induced fibrosis. For clarity, the term interstitial pneumonia (IP) will be used for the remainder of this chapter.
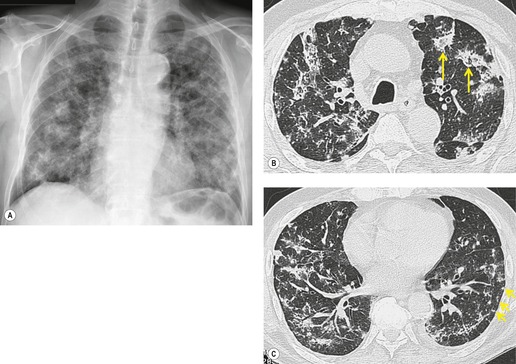
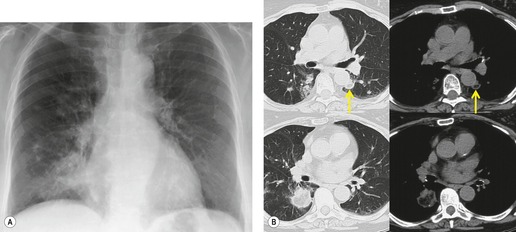
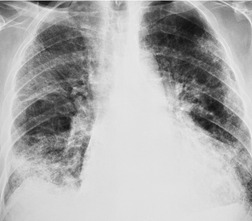
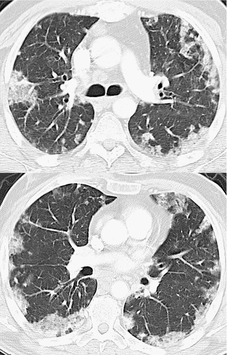
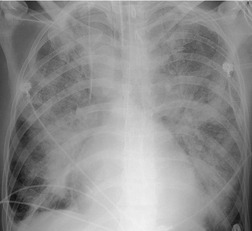
Although many drugs may cause interstitial pneumonia,11. and 12. the drugs most frequently associated are amiodarone, bleomycin, etoposide, gemcitabine, taxane derivatives (docetaxel, paclitaxel), methotrexate, and carmustine (see Table 9.1).10.25.26.27.45.46.47.48. and 49. Gold salts and chlorambucil are less common causes of drug-induced interstitial pneumonia. 26 Drug-induced interstitial pneumonia manifests on chest radiographs with heterogeneous opacities that are either diffuse or basal in distribution (Fig. 9.6).26.27.48. and 50. HRCT shows scattered areas of ground-glass opacity, focal areas of consolidation, and irregular linear opacities, often in a basal and peribronchovascular distribution. 51
Organizing pneumonia
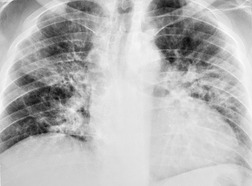
Organizing pneumonia (OP) is a term used to describe inflammatory infiltration and fibrosis that predominantly involves the airspaces. Organizing pneumonia is characterized histopathologically by fibroblastic plugs (Masson bodies) within the respiratory bronchioles, alveolar ducts, and adjacent alveolar spaces, and accumulation of lipid-laden macrophages in the distal airspaces. 52 The drugs most commonly implicated are bleomycin, doxorubicin, methotrexate, cyclophosphamide, and gold salts (see Table 9.1).1.10.15.25.26. and 45. Amiodarone, nitrofurantoin, penicillamine, thalidomide, topotecan and sulfasalazine are less common causes of drug-induced organizing pneumonia. 1 Organizing pneumonia manifests on chest radiographs with rounded, scattered, and often peripheral areas of consolidation (Fig. 9.7).26.27. and 50. HRCT usually shows bilateral scattered areas of ground-glass opacification or consolidation that are often peripheral in distribution. Centrilobular nodules and branching linear opacities are common associated findings. 52
LUNG INJURY DUE TO HYPERSENSITIVITY REACTIONS
The molecular size of most drugs is too small to directly provoke an immune response, therefore it is likely that they act as haptens in combination with endogenous protein.53. and 54. The provoked immune responses are most commonly type I (immediate hypersensitivity) or type III (immune complex) reactions. Drugs most commonly associated with hypersensitivity reactions are shown in Box 9.3. Hypersensitivity reactions are usually immediate and may become clinically apparent within hours or days after institution of drug therapy (Fig. 9.8). Fever and peripheral eosinophilia suggest the diagnosis. Withdrawal of the offending drug and administration of corticosteroids usually results in prompt and complete resolution of symptoms. Hypersensitivity reactions to drugs may predominantly affect the airways or the lung parenchyma.14. and 18. Airway hypersensitivity results in bronchial constriction, mucus hypersecretion and inspissation, and mucosal edema with eosinophilic infiltration. This clinically manifests as drug-induced asthma and lacks specific radiographic features. 18
Box 9.3
• Antiarthritics
• Aurothioglucose (gold salts)
• Antiasthmatic
• Sodium cromoglycate
• Leukotriene antagonists (montelukast, zafirlukast)
• Antibacterials
• Erythromycin
• Nitrofurantoin
• Para-aminosalicylic acid
• Isoniazid
• Penicillin
• Sulfonamides
• Antidepressants
• Imipramine
• Antihypertensives
• Mecamylamine
• Hydralazine
• Central nervous system (CNS) stimulants
• Methylphenidate
• Chemotherapeutic agents
• Bleomycin
• Etoposide
• Gemcitabine
• Methotrexate
• Teniposide
• Trastuzumab
• Oxaliplatin
• Procarbazine
• Azathioprine
• Paclitaxel
• Hypoglycemic agents
• Chlorpropamide
• Muscle relaxant
• Dantrolene
• Chelating agents
• Penicillamine
• Anti-inflammatory agents
• Sulfasalazine
• Nonsteroidal anti-inflammatory drugs
Pulmonary parenchymal involvement can result in either acute or chronic eosinophilic pneumonia. 55 Although many drugs can cause eosinophilic pneumonia, the agents most commonly implicated are penicillamine, sulfasalazine, nitrofurantoin, para-aminosalicylic acid, and nonsteroidal anti-inflammatory drugs (see Table 9.1).1.14.26.45.46.56.57.58.59.60.61. and 62. Eosinophilic pneumonia is characterized histopathologically by accumulation of eosinophils and macrophages in alveolar spaces. 46 There is usually an accompanying infiltrate of eosinophils, lymphocytes, and plasma cells within alveolar septa and adjacent interstitium. Eosinophilia is often present in the peripheral blood. Drug-induced eosinophilic pneumonia manifests on chest radiographs with scattered areas of subsegmental consolidation, often in the periphery of the lung (Fig. 9.9). The extent of consolidation varies and distribution within the peripheral lungs is random. The opacities tend to be fleeting – resolving in one area of the lung only to reappear in another. HRCT shows ground-glass opacities and consolidation that is typically peripheral and upper lobe in distribution (Fig. 9.9). 63
A hyperacute hypersensitivity reaction has been described in patients receiving bleomycin,64. and 65. methotrexate,66. and 67. and carbemazapine68– particularly after previous exposure to the drug. In these cases, the patient presents with acute onset of respiratory symptoms and chest radiographs show diffuse heterogeneous or homogeneous opacities. Response to discontinuation of the offending drug is usually prompt, but a similar reaction after readministration of the drug may be expected.
LUNG INJURY DUE TO NEURAL OR HUMORAL MECHANISMS
Drugs are a common cause of noncardiogenic pulmonary edema, often exerting their effect by altering capillary permeability.16.69. and 70. Tocolytic agents used to arrest premature labor, such as terbutaline or isoxsuprine, can induce pulmonary edema. 16 While not a drug in the strict sense of the word, blood transfusions can induce severe pulmonary edema (transfusion-related acute lung injury). 17 Both inhaled and injected opioids and other CNS depressants cause neurogenic pulmonary edema. In such cases, pulmonary capillary permeability is altered, either by direct toxic action on the lung or by impulses from the brainstem and hypothalamus,71.72. and 73. and fluid leaks into the pulmonary interstitium and alveoli. However, not all cases of drug-induced pulmonary edema operate through these mechanisms. Some drugs cause water and salt retention, leading to fluid imbalance and edema. Edema caused by drug toxicity is indistinguishable from other forms of edema both in its radiographic appearance and in the rapidity of onset and clearing (Fig. 9.10). Drugs most commonly associated with pulmonary edema are shown in Box 9.4.
Box 9.4
• Analgesics
• Acetylsalicylic acid
• Codeine
• Pentazocine
• Antibacterials
• Nitrofurantoin
• Antidepressants
• Amitriptyline
• Doxepin
• Desipramine
• Imipramine
• Anti-inflammatories
• Phenylbutazone
• Oxyphenbutazone
• β-Adrenergic agonists
• Albuterol
• Isoxsuprine
• Ritodrine
• Terbutaline
• Chemotherapeutic agents
• All-trans-retinoic acid (ATRA)
• Cyclophosphamide
• Gemcitabine
• Imatinib
• Taxanes
• Methotrexate
• Cytosine arabinoside
• Diuretics
• Hydrochlorothiazide
• Immune suppressants
• Interleukin-2
• OKT3
• Miscellaneous
• Iodinated contrast media
• Dextran
• Colchicine
• Epinephrine
• Opiates
• Heroin
• Propoxyphene
• Methadone
• Cocaine (crack)
• Organophosphate insecticides
• Parathion
• Malathion
• Phenothiazines
• Chlorpromazine
• Sedatives
• Chlordiazepoxide
• Ethchlorvynol
Drug-induced asthma may also result from neurohumoral mechanisms. 18 Certain drugs, including β-adrenergic antagonists such as propranolol and parasympatheticomimetics such as neostigmine, cause asthma by directly affecting bronchial innervation. Aspirin may cause asthma by inhibiting prostaglandin synthesis. 74
LUNG INJURY DUE TO AUTOIMMUNE MECHANISMS
Drug-induced lupus
Many drugs can trigger an autoimmune response and cause systemic lupus erythematosus.75.76. and 77. At least 90% of cases of drug-induced lupus are associated with procainamide, hydralazine, isoniazid, or phenytoin. Antinuclear antibodies are usually detectable in the serum of affected patients and are often found in patients taking these drugs in the absence of clinical or radiographic evidence of lupus. Drugs most commonly associated with systemic lupus erythematosus are shown in Box 9.5.
Box 9.5
• Antibacterials
• Griseofulvin
• Isoniazid
• Nitrofurantoin
• Para-aminosalicylic acid
• Penicillin
• Streptomycin
• Sulfonamides
• Anticonvulsants
• Carbamazepine
• Ethosuximide
• Methsuximide
• Phenytoin
• Primidone
• Trimethadone
• Anti-inflammatories
• Phenylbutazone
• Oxyphenbutazone
• Cardiovascular agents
• Clofibrate
• Digitalis
• Hydralazine
• Methyldopa
• Procainamide
• Propranolol
• Reserpine
• Quinidine
• Diuretics
• Chlorthalidone
• Thiazides
• Thyroid blockers
• Thiouracil
• Propylthiouracil
• Miscellaneous
• Phenothiazines
• Penicillamine
• Methysergide
• Gold salts
• Levodopa
Drug-induced lupus does not differ in its pleural or pulmonary manifestations from the idiopathic form of the disease. Pleural effusion is by far the most common radiographic manifestation of this condition and there may be a concomitant pericardial effusion (Fig. 9.11). Lung parenchymal involvement is uncommon but, when it occurs, typically manifests with bilateral basal heterogeneous opacities. As some of the drugs that cause this condition are used to treat cardiac disease, and because the manifestations of cardiac disease can be similar to those of drug-induced lupus, diagnosis can be difficult and is often delayed.
Drug-induced vasculitis
Many drugs are associated with development of small-vessel lung vasculitis (Box 9.6).20.78. and 79. Drug-induced vasculitis may be an expression of a type III (immune complex) or a type IV (cell-mediated) hypersensitivity response. Pulmonary involvement is usually associated with, and often overshadowed by, systemic vasculitis that particularly involves the skin, kidneys, and liver. Drug-induced pulmonary vasculitis can result in pulmonary infarction and alveolar hemorrhage. Diagnosis of drug-induced pulmonary vasculitis usually requires evidence of vasculitis on lung biopsy and a history of appropriate drug exposure. Churg–Strauss syndrome, a form of granulomatous vasculitis, has been reported to develop in asthmatic patients receiving leukotriene antagonists. 20 Granulomatous vasculitis can also develop in intravenous drug users for reasons discussed below.
Box 9.6
• Antiasthmatics
• Sodium cromoglycate
• Leukotriene antagonists (montelukast, zafirlukast)
• Antibacterials
• Sulfonamides
• Penicillin
• Anticonvulsants
• Phenytoin
• Anti-inflammatories
• Phenylbutazone
• Cardiovascular agents
• Quinidine
• Hydralazine
• Chemotherapeutic agents
• All-trans-retinoic acid (ATRA)
• Busulfan
• Mitomycin
• Thyroid blockers
• Thiouracil
• Propylthiouracil
• Tranquillizers
• Phenothiazines
Imaging findings of drug-induced pulmonary vasculitis are variable and nonspecific. Scattered areas of subsegmental consolidation in a peripheral distribution may occur in a pattern similar to that seen in cases of drug-induced eosinophilic pneumonia. In other patients, more diffuse heterogeneous or homogeneous opacities are seen. Cavitation may occur in areas of infarction. Pleural effusion is rare.
DRUG-INDUCED PULMONARY GRANULOMATOSIS
Granulomas are aggregates of pulmonary macrophages that are reacting to certain microorganisms, foreign particles, various drugs such as methotrexate and nitrofurantoin, or other unknown stimuli. A number of granulomatous pulmonary reactions are related to drug therapy, including granulomatous vasculitis, granulomatous pneumonitis, and exogenous lipid pneumonia (Box 9.7).
Box 9.7
• Granulomatous vasculitis (‘drug-abuser’s lung’)
• Intravenous talc injection
• Intravenous starch injection
• Intravenous cellulose injection
• Granulomatous pneumonitis or sarcoidlike reaction
• Nitrofurantoin
• Sirolimus
• Interferon
• Methotrexate
• Exogenous lipid pneumonia
• Mineral oil
• Oily nasal drops
• Occupational exposure
Granulomatous vasculitis
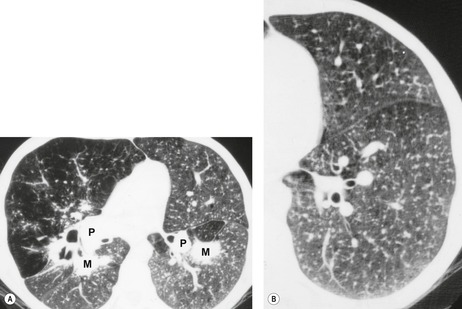
Intravenous administration of illicit drugs can cause a granulomatous vasculitis and pulmonary hypertension (drug abuser’s lung). Vasculitis in these cases is caused by a foreign body giant cell reaction to contaminants or suspending agents such as talc, starch, or cellulose that embolize the lungs.80. and 81. Distinctive pathologic features include: medial hypertrophy of muscular pulmonary arteries with thrombosis and recanalization; and foreign body giant cell granulomas containing doubly refractile particles in the lumina and arterial walls, in the perivascular connective tissues and in the alveolar septa. Patients may be asymptomatic when the condition is discovered. Many eventually progress to severe respiratory insufficiency. Typical radiographic and imaging findings include pulmonary hyperinflation, diffuse small nodular opacities, large bulla, and vascular and cardiac changes of pulmonary hypertension (Fig. 9.12). With time, the nodules may enlarge and coalesce into large, often perihilar conglomerate masses (progressive massive fibrosis) in the mid- to upper lungs, similar to those seen in cases of silicosis.82.83.84.85.86.87. and 88.
Granulomatous pneumonitis
A variety of drugs, including cocaine, methotrexate, nitrofurantoin, and sirolimus, have been reported to result in a granulomatous interstitial pneumonitis that resembles sarcoidosis. 10 Features suggestive of hypersensitivity pneumonitis are sometimes seen in these cases as well.
Sarcoidosis is a granulomatous disease of unknown etiology. The pathologic hallmark of sarcoidosis is the presence of interstitial noncaseating granulomas that typically involve the lymphatics of the bronchovascular bundles. While the exact pathogenesis of sarcoidosis remains in doubt, a number of immune modulators have been implicated, including interferons. Recent reports suggest that interferon therapy for malignant and nonmalignant conditions (such as hepatitis) may cause a sarcoidlike reaction.89.90.91. and 92. In the largest series to date, 7% of patients treated with interferon-2α developed pulmonary disease resembling sarcoidosis that regressed with discontinuation of interferon. 89 Prognosis for interferon-induced sarcoidosis seems to be good, provided that the medication can be discontinued. A sarcoidlike reaction induced by methotrexate therapy has also been reported.93. and 94.
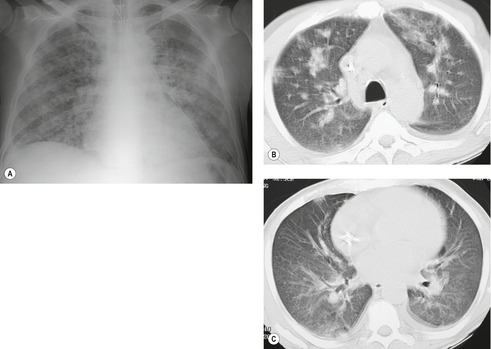
The radiographic and imaging findings of drug-induced sarcoidlike reactions are similar to those described for idiopathic sarcoid: hilar and mediastinal lymphadenopathy, fine nodular opacities (miliary pattern), reticulation, or, less commonly, airspace opacities (Fig. 9.13).89. and 95. These findings typically predominate in the mid- to upper lungs. CT frequently demonstrates a distinctly bronchovascular distribution of abnormalities.
Exogenous lipid pneumonia
Exogenous lipid pneumonia (ELP) is a fairly common form of granulomatous pneumonitis that results from chronic aspiration of mineral oils or other lipids. Because mineral oil is inert and is not hydrolyzed by lipase, it causes a foreign body giant cell reaction in the lung. ELP most frequently occurs in elderly patients with swallowing disorders or hiatal hernias and who use mineral oil as a laxative or oily nose drops. It can also result from occupational exposure to oil mist. Because patients regard the use of mineral oils as something normal and inconsequential, they often fail to volunteer this information. Thus, the diagnosis of ELP can be difficult. Although the diagnosis can sometimes be suggested by imaging, it is more often made by pathologic examination of affected lung. Staining of fluid retrieved from bronchoalveolar lavage for neutral fats can also be diagnostic.
Most patients with ELP are asymptomatic. In these cases, the condition is discovered on ‘routine’ chest radiographs. A few patients complain of chronic cough or chest pain. Longstanding, recurrent aspiration can result in fibrosis that manifests with severe, progressive dyspnea. Three patterns have been described on chest radiographs. First, acute aspiration of a large volume of oil can result in diffuse airspace consolidation. Affected patients are typically quite ill and have marked respiratory distress. This type of ELP is fortunately quite rare. Second, chronic low-grade aspiration of oil over a long period of time can result in either focal or multifocal homogeneous opacities or consolidation (Figs 9.14 and 9.15). Affected patients can be asymptomatic despite impressive radiographic abnormalities and late fibrosis can result. Third, aspiration of oil can result in one or more pulmonary masses (Fig. 9.16). Affected patients are also typically asymptomatic and differentiation from bronchogenic carcinoma can be difficult.96.97. and 98. Cavitation is uncommon in cases of ELP and, when present, is usually due to superinfection by atypical mycobacteria, particularly Mycobacterium fortuitum or Mycobacterium chelonae.
On CT, ELP can manifest with airspace consolidation or irregular masslike opacities. Superimposed fibrosis with irregular linear opacities, traction bronchiectasis, and honeycombing can also be seen. A distinctive and frequently diagnostic finding of ELP on CT is fat within the consolidation or mass (Figs 9.15 and 9.16). The ‘crazy-paving’ appearance on HRCT has also been described in patients with ELP (Fig. 9.17). 99
MISCELLANEOUS FORMS OF DRUG-INDUCED LUNG INJURY
Drug-induced alveolar hemorrhage
Diffuse alveolar hemorrhage can result from a drug-induced small-vessel vasculitis (see above), direct lung toxicity, or as a consequence of a drug-induced coagulopathy. 20 Diffuse alveolar hemorrhage is a relatively uncommon complication of drug therapy. Drugs most commonly associated with diffuse alveolar hemorrhage are amphotericin B, high-dose cyclophosphamide, mitomycin, cytarabine (Ara-C), all-trans-retinoic acid, and penicillamine1.26. and 45. (see Table 9.1). Penicillamine can cause a syndrome of pulmonary hemorrhage with or without renal failure. 100 Penicillamine-induced pulmonary hemorrhage is thought to be an immune-complex disorder. Affected patients are typically dyspneic and have a falling hematocrit. Hemoptysis may or may not occur. Mitomycin therapy causes a hemolytic–uremic syndrome in 2–10% of patients and typically occurs 4–8 months after a course of therapy. Affected patients manifest with diffuse hemorrhage and renal failure that can progress to acute respiratory distress syndrome (ARDS). 20 Anticoagulant therapy can result in diffuse alveolar hemorrhage for obvious reasons, although these agents are often overlooked. 101
Chest radiographs in patients with drug-induced alveolar hemorrhage show scattered airspace consolidation of variable severity that is generally rapid in onset and clears fairly quickly. HRCT usually shows bilateral, scattered or diffuse, ground-glass opacities (Fig. 9.18). 102 There are no associated hilar or mediastinal abnormalities. Drug-induced alveolar hemorrhage is usually an isolated event; fibrosis, as is seen in patients with idiopathic pulmonary hemosiderosis or Goodpasture syndrome and repeated episodes of hemorrhage, is uncommon. Bronchoalveolar lavage that yields increasingly bloody aliquots of fluid is characteristic.
Drug-induced pulmonary hypertension
Drug-induced pulmonary hypertension appears to be rare. 19 The first documented instance of this complication occurred in the 1960s when aminorex, an over-the-counter appetite suppressant, was marketed in Europe. Pulmonary arteriolar vasospasm induced by aminorex caused pulmonary arterial hypertension and cor pulmonale. 103 More recently several amphetamine-like appetite-suppressant drugs, particularly the fenfluramines, have been implicated in cases of pulmonary arterial hypertension.104.105. and 106. These drugs may also cause valvular heart disease involving both right- and left-sided valves. 107 Late-onset pulmonary hypertension has also been reported in cases of Spanish toxic oil syndrome and in patients who used l-tryptophan preparations. 19
Drug-induced pulmonary thromboembolism
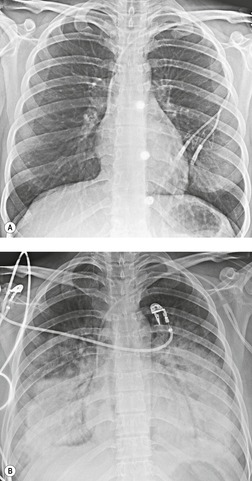
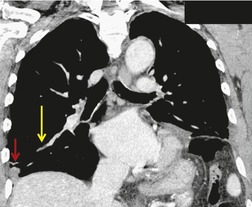
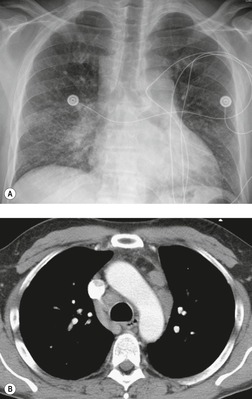
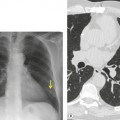
Venous thromboembolism is associated with the use of oral contraceptives and hormone replacement therapy.108.109. and 110. Randomized trials have shown a two- to threefold increased risk of venous thromboembolism in postmenopausal women treated with hormone replacement therapy.108. and 109. Although the risk is highest in the first year of treatment, increased risk persists after the first year if treatment continues. 109 Risk increases significantly in patients with various thrombophilic conditions such as factor V Leiden deficiency or a family history of thrombosis. 111 A recently introduced cancer therapy directed against vascular endothelial growth factor, bevacizumab (Avastin), has been associated with an increased risk of deep venous thrombosis and pulmonary embolism (Fig. 9.19). 25 Patients treated with thalidomide or lenalidomide, agents used to treat multiple myeloma, are also at increased risk for thromboembolic events. 25
Heparin-induced thrombocytopenia is very strongly associated with thrombotic events, including pulmonary embolism. While heparin-induced thrombocytopenia most frequently occurs during treatment with unfractionated heparin, it can also occur with low-molecular-weight heparins, but at a lower rate of frequency.112.113. and 114.
Oil embolism occurs to some extent in all properly conducted lymphangiography examinations. Only when patients have poor pulmonary reserve or when excessive amounts of the contrast medium are administered is there any potential for harm. For this reason, patients with poor respiratory function should not undergo lymphangiography. Chest radiographs obtained 24–48 hours after administration of the contrast medium may show a very fine nodular (miliary) pattern. At the same time, the patient may experience mild fever and slight shortness of breath on exertion. These clinical and radiographic findings are usually transient. 115
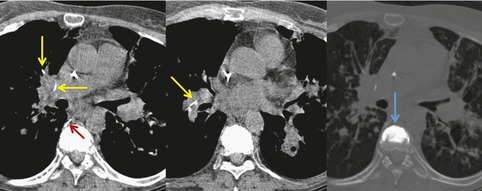
Vertebroplasty and kyphoplasty are procedures recently introduced for treatment of malignant and osteoporotic spinal compression fractures.116. and 117. These procedures involve percutaneous injection of cement, usually polymethylmethacrylate, into the fractured vertebral body. Because the material is injected under pressure, cement can leak into the paravertebral veins, gain access to the systemic venous system and embolize to the lungs (Fig. 9.20).116.117.118.119.120.121. and 122. Pulmonary cement embolism occurs in up to 7% of treated patients and is more common in treatment of malignant rather than benign fractures.116.117. and 122. Although affected patients are often asymptomatic, severe pulmonary compromise or even respiratory failure and death have been reported. 119 On chest radiographs or CT, these emboli are seen as high-attenuation branching structures in the pulmonary arteries. Cement visualization in the vertebral bodies, or the paravertebral or systemic veins, is a suggestive finding.
Drug-induced obliterative bronchiolitis
Obliterative bronchiolitis is a process that results in permanent, fibrous occlusion of small airways. It is an important manifestation of chronic graft-versus-host disease in bone marrow transplant recipients and of chronic rejection in lung transplant recipients (see Chapter 6). Obliterative bronchiolitis can also be the result of toxic fume inhalation and is a rare manifestation of pulmonary drug toxicity. Penicillamine18.123.124. and 125. and consumption of uncooked leaves of bush tea (Sauropus androgynus) 126 have been reported to cause obliterative bronchiolitis. Radiographic and imaging findings of obliterative bronchiolitis are more fully discussed in Chapters 4 and 12 and include pulmonary hyperinflation, mosaic attenuation on inspiratory HRCT, lobular air-trapping on expiratory CT, and bronchiectasis.127.128.129. and 130.
Drug-induced pulmonary calcification
Pulmonary calcification usually occurs in the setting of an abnormal calcium–phosphorus product (typically greater than 70 mg2/dL2). Alkaline environments favor calcium deposition; hence the lung apices are more commonly affected than the bases. Calcium predominately deposits in alveolar and bronchial walls and in the walls of pulmonary vessels. When severe, calcification can produce fibrosis or invoke a foreign body response. The most frequent cause of pulmonary parenchymal calcification is hyperparathyroidism. Pulmonary calcification can also occur after prolonged vitamin D and calcium therapy and in the milk–alkali syndrome. Most patients with metastatic pulmonary calcification are asymptomatic, a minority have nonspecific respiratory complaints, and a few develop lethal respiratory distress. Chest radiographs are usually normal. When abnormal, bilateral confluent nodular opacities or consolidation is seen (Fig. 9.21). The calcific nature of the opacities is often not evident on the chest radiograph. The radiographic pattern can be mistaken for pneumonia or pulmonary edema.
CT, especially HRCT, can detect pulmonary involvement when the chest radiograph is normal. 131 The most common findings of metastatic pulmonary calcification on HRCT are 3–10 mm diameter, poorly marginated, centrilobular nodules (Fig. 9.22).132.133. and 134. Many, though not all, nodules are of increased attenuation on CT or may be frankly calcified. Septal thickening and irregular linear opacities are uncommon. CT can also show calcification in chest wall vessels, a helpful adjunctive finding. Up to 60% of patients with chronic renal failure and a normal chest radiograph have pulmonary uptake on Tc-99m bone scintigraphy, a finding that is considered diagnostic of metastatic pulmonary calcification.135. and 136. Diagnosis can also be made at lung biopsy. There is a single case report of magnetic resonance imaging (MRI) of pulmonary calcinosis. 137 Unexpectedly, high signal intensity was noted on T1-weighted images. This was explained by more rapid relaxation of water protons on the surface of diamagnetic calcium crystals.
Drug-related pleural effusion and fibrosis
Drug-induced pleural effusion usually occurs in the setting of the systemic lupus erythematosus syndrome.21. and 138. Over 50% of patients with drug-induced lupus develop pleural effusions at some stage of their illness. Effusions can also occur in the setting of hypersensitivity reactions to nitrofurantoin,7.139. and 140. methotrexate, 141 and procarbazine. 142 Such effusions can have elevated levels of pleural eosinophils and may resolve without sequelae. 21 However, treatment with methysergide is associated with both pleural fluid and pleural fibrosis. 143 Irregular masses of pleural fibrosis may be interspersed with loculated pleural fluid and there may be associated mediastinal fibrosis. Ergotamine, ergonovine maleate, bromocriptine, amiodarone, 144 and pergolide can produce similar effects.145. and 146.
Drug-related lymphadenopathy
A few drugs are known to cause hilar or mediastinal lymphadenopathy, including phenytoin and methotrexate. Phenytoin can cause a pseudolymphoma syndrome with generalized lymphadenopathy, fever, skin rashes, eosinophilia, and hepatosplenomegaly (Fig. 9.23). 147 There is a slightly increased risk of lymphoma in patients taking this drug. Lymphadenopathy in the setting of methotrexate therapy is frequently granulomatous in nature and may represent a form of drug-induced sarcoidosis (see above).93.94. and 148. Since methotrexate is used in the treatment of malignancy, the possibility of mistaking methotrexate-induced lymphadenopathy for metastatic disease is real.
Drug-induced infection
Infection is a common untoward side effect of many immunosuppressive agents used to treat cancer or nonmalignant inflammatory conditions, such as rheumatoid arthritis, vasculitis, and inflammatory bowel disease. 149 Corticosteroids are the most commonly used immunosuppressive agents and predispose to all types of infections, but to Pneumocystis pneumonia, invasive aspergillosis, and tuberculosis in particular. The recently introduced antitumor necrosis factor α (anti-TNFα) agents, etanercept and infliximab, pose a significant risk for reactivation tuberculosis and disseminated histoplasmosis. 149 Patients receiving methotrexate, particularly for rheumatoid arthritis, are at particular risk for infections due to Cryptococcus, Nocardia, and Pneumocystis.149 Drugs used to treat human immunodeficiency virus (HIV) infection, the so-called highly active antiretroviral agents, may cause reactivation of latent mycobacterial or fungal infection as a manifestation of the immune restoration syndrome.150. and 151.
SPECIFIC DRUGS AND THEIR ADVERSE EFFECTS
Certain drugs are discussed here in further detail because their adverse effects are relatively common or serious, because their adverse effects have noteworthy features, or because the agents are relatively new. As noted above, the number of new drugs with reported lung toxicity increases almost daily. The interested reader is referred to several recent reviews24. and 25. and the excellent online resource, www.pneumotox.com,8 for more in-depth and up-to-date information.
All-trans retinoic acid
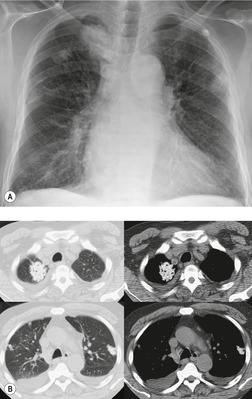
All-trans retinoic acid induces maturation of leukemic cells in patients with acute promyelocytic leukemia. Between 15% and 25% of treated patients152. and 153. develop ‘retinoic acid syndrome’. This syndrome, which develops within 5–15 days of initiation of therapy, is characterized by fever, respiratory distress, generalized edema, cardiac decompensation, and hypotension. The etiology is uncertain, but is probably related to lung tissue infiltration by maturing leukocytes that release vasoactive cytokines, causing increased capillary permeability. Retinoic acid syndrome is usually associated with a rise in the white blood cell count to over 20 000 cells/mm3 and its severity correlates with the peak white blood cell count. 153 It is potentially fatal although prompt treatment with corticosteroids results in resolution of symptoms.153. and 154. Chest radiographs in affected patients usually show diffuse heterogeneous or homogeneous opacities and pleural effusions.152. and 153.
Amiodarone (see Box 9.8)
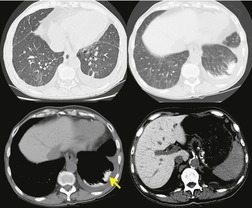
Amiodarone, an iodinated benzofuran derivative, is used to treat a variety of cardiac rhythm disturbances. 13 Amiodarone contains 37% iodine by weight, is concentrated in the lung and liver, and has a relatively long tissue half-life. This may account for the relatively delayed appearance of pulmonary toxic effects after commencement of therapy (median 6 months) and slow resolution after cessation (median 3 months). 155 Furthermore, the latent period between beginning drug therapy and onset of signs and symptoms of toxicity is variable, and may occur several years later. Toxicity from amiodarone appears to be dose related.34.48. and 156. The frequency of amiodarone toxicity ranges from 5% to 10% in patients treated with high doses (>400 mg daily) and is approximately 2% in patients treated with low doses of amiodarone (≤400 mg). The risk of lung injury is also increased in elderly patients34 and in patients receiving high levels of inspired oxygen during and after surgery.43. and 157. For instance, patients treated preoperatively with amiodarone have an increased risk of acute respiratory distress syndrome after cardiac surgery. 158
Box 9.8
• Commonly used for treatment of:
• Ventricular arrhythmias
• Atrial arrhythmias (increasingly common)
• Toxicity
• Risk increases with dose >400 mg/day
• Toxicity does occur at lower doses (200 mg/day)
• Histopathology
• Interstitial pneumonia, diffuse alveolar damage most common
• Organizing pneumonia less common
• ‘Foamy’ macrophages suggest exposure to drug, not necessarily toxicity
• Radiographic and imaging findings
• Usually nonspecific, but include diffuse opacities resembling pulmonary edema, diffuse reticular opacities or multifocal consolidation (organizing pneumonia)
• High-attenuation pleural or parenchymal opacities suggestive, but neither diagnostic nor very common
• Increased liver, spleen attenuation indicates exposure
• Occasional pleural effusion or thickening
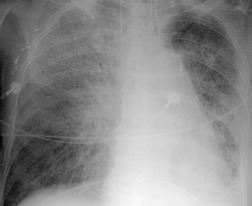
Interstitial pneumonia is the most common histopathologic manifestation of amiodarone-induced lung disease; diffuse alveolar damage and organizing pneumonia are less common (see Fig. 9.7).50. and 159. Amiodarone toxicity is somewhat unusual in that a specific histopathologic finding (intraalveolar accumulation of macrophages with foamy cytoplasm) is considered characteristic. However, such macrophages can also be found in asymptomatic patients receiving amiodarone who have no clinical or radiographic and imaging evidence of disease. Thus, this ‘characteristic’ histopathologic finding may indicate nothing more than exposure to the drug. The commonest presenting complaint of amiodarone toxicity is dyspnea. Less common complaints include pleuritic chest pain, cough, fever, and weight loss. 160 DLco is usually reduced. 156 As is the case with many drugs, chest radiographs may be normal in some patients with early signs or symptoms of amiodarone toxicity. In this setting, HRCT can be useful for showing findings of early lung injury. 161
The chest radiographic findings of more advanced amiodarone toxicity are quite variable. Chest radiographs of affected patients frequently show multiple peripheral areas of consolidation – findings that resemble those seen in patients with hypersensitivity reactions (Figs 9.24 and 9.25). However, there is usually no evidence of either blood or tissue eosinophilia. Less commonly, amiodarone toxicity manifests with diffuse heterogeneous opacities (Fig. 9.26), focal or multifocal homogeneous opacities (Fig. 9.27), or scattered nodular opacities on chest radiographs. Pleural effusions can occur in association with manifestations of lung injury due to development of pleural inflammation (Fig. 9.28). 162
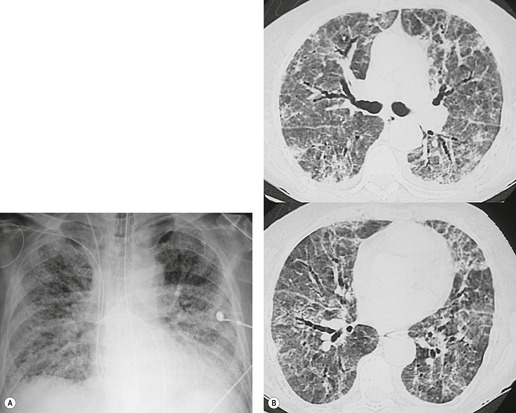 |
| Fig. 9.26 (Courtesy of Dr. Santiago Rossi, Buenos Aires, Argentina.) |
As patients treated with amiodarone usually have major cardiac disease, the clinical and radiographic findings of amiodarone toxicity can be difficult to distinguish from the pulmonary edema of heart failure. Lack of response to treatment for heart failure and lack of short-term fluctuations in pulmonary opacities suggests amiodarone toxicity. Since amiodarone contains iodine by weight and is concentrated in the liver and lung, CT attenuation of these tissues may be consequently increased in patients receiving the drug.41.42.44. and 163. As is the case with finding foamy macrophages on histopathologic specimens, increased attenuation on CT is likely more a marker of drug exposure than toxicity. Nevertheless, the finding of high-attenuation pleural or parenchymal lesions and increased liver attenuation on CT suggests the diagnosis of amiodarone toxicity in the appropriate clinical setting (Fig. 9.28).41. and 44.
Amiodarone toxicity is potentially fatal if untreated, but may be reversible if diagnosed and treated early. 164 Stringent monitoring of patients receiving amiodarone therapy is therefore essential. Sunderji et al. 164 recommend that baseline chest radiographs and pulmonary function tests should be carried out prior to institution of amiodarone therapy. They further recommend that follow-up radiographs at 3–6-month intervals be obtained, but suggest that repeat lung function testing or CT should be reserved for patients who develop new symptoms or radiographic abnormalities.
Recently, low-dose amiodarone (<400 mg daily) has gained popularity for treating refractory atrial arrhythmias, including atrial fibrillation. 165 Although the frequency of toxicity in these patients is considerably less than that of patients receiving higher doses, it is not insignificant (Figs 9.27 and 9.29).159.160.164.165.166. and 167. Because these patients are on low-dose therapy, however, the association between symptoms, radiographic and imaging abnormalities, and amiodarone may be overlooked. Ott et al. 166 reported eight patients on low-dose therapy who developed signs and symptoms of toxicity. Seven patients responded to cessation of therapy and administration of corticosteroids, but one died of progressive respiratory failure.
Bacille Calmette Guérin (BCG)
Intravesical BCG therapy is used to treat superficial bladder cancer. Both early and late complications of BCG therapy have been reported.168.169.170.171. and 172. Early complications generally occur within 3 months of instillation and are probably due to a hypersensitivity reaction.173. and 174. Affected patients present with constitutional complaints, hepatitis, 175 diffuse pneumonitis, or pericarditis. Noncaseating granulomas are found on biopsy in most cases. Chest radiographs or CT may be normal or show either diffuse heterogeneous or fine nodular opacities (miliary pattern) (Fig. 9.30).172.176.177.178.179. and 180. Treatment includes antituberculous drugs and, in cases where features of hypersensitivity predominate, corticosteroids.170. and 181. Prognosis is generally good, although fatalities are reported.182.183.184. and 185. Diffuse alveolar damage is rarely reported as a complication of BCG therapy. 186 Late complications occur more than a year after BCG treatment. Disease usually manifests as focal mycobacterial infection of the genitourinary tract. Infection at other sites such as the spine, kidneys, or retroperitoneal soft tissues can also occur. 170
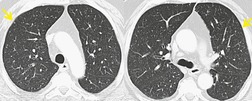 |
| Fig. 9.30 (Courtesy of Dr. Jeremy Erasmus, Houston, TX, USA.) |
Bleomycin (Box 9.9)
The cytotoxic antibiotic bleomycin is used to treat a variety of neoplasms, including squamous cell carcinoma, lymphoma, and testicular malignancies. Bleomycin is concentrated in the lung, which is therefore a primary target for adverse effects. Toxicity is related to the cumulative dose. Risk of toxicity increases considerably when doses exceed a total of 450 IU.12. and 187. The reported frequency of overt pulmonary toxicity varies from 4%65 to 15%. 188 The risk and severity of pulmonary toxicity increases with age, prior or concomitant radiation therapy, concomitant treatment with other chemotherapeutic agents, oxygen therapy, decreased renal function, or bleomycin readministration within 6 months of discontinuation.27. and 188. On the other hand, thalidomide has been shown to reduce frequency of bleomycin toxicity, at least in animal models. 189 Diffuse alveolar damage is the most common histopathologic manifestation of bleomycin-induced lung toxicity (see Fig. 9.3); interstitial inflammation and organizing pneumonia are less common manifestations. 28 An acute hypersensitivity reaction to low doses of bleomycin has also been reported. 27
Box 9.9
• Commonly used for treatment of:
• Germ cell neoplasms
• Kaposi sarcoma
• Lymphoma
• Squamous cell carcinoma
• Toxicity
• Risk increases with total dose >450 IU
• Risk enhanced by oxygen therapy
• Histopathology
• Diffuse alveolar damage common
• Interstitial pneumonia and organizing pneumonia less common
• Radiographic and imaging manifestations
• Early chest radiograph – basal reticulonodular opacities in costophrenic angles
• Early CT – basal ground-glass opacities, subpleural linear opacities (prone imaging useful)
• Late – progressive fibrosis, loss of lung volume
• Uncommon – pulmonary nodules simulating metastases
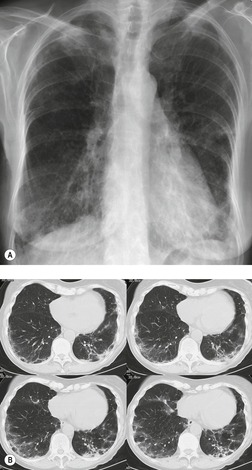
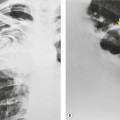
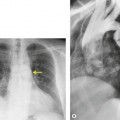
Bleomycin toxicity can be quite serious, as mortality rates up to 25% have been reported. However, early detection may result in a substantial improvement in prognosis because mild injury may respond to discontinuation of bleomycin and corticosteroid administration. 2 The earlier the toxic effects are detected, the greater the likelihood of a favorable response to treatment. Hence strict surveillance of patients under treatment with bleomycin, as with all patients undergoing chemotherapy, is imperative. Bleomycin toxicity ordinarily becomes apparent within 3 months of initiation of therapy and manifests clinically with progressive dyspnea and cough. 2 Reduction in DLco appears to be the most sensitive means for detecting early lung toxicity. However, reduction in DLco may occur in patients receiving bleomycin who never develop clinically apparent toxicity. 190 The chest radiograph is often normal in early toxicity despite a decreased DLco (Fig. 9.31), abnormal lung accumulation of gallium-67, ground-glass opacities on HRCT, or abnormal bronchoalveolar lavage fluid or lung biopsy findings.191. and 192.
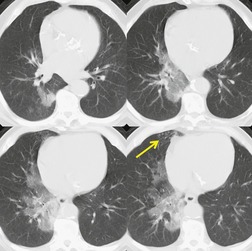
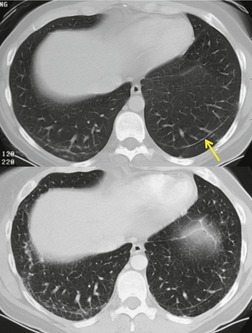
The earliest findings of bleomycin toxicity on chest radiographs are basal reticulonodular opacities (Figs 9.32 and 9.33). Balikian et al. 193 noted that the earliest findings consistently appeared in the costophrenic sulci and in the subpleural regions of the lungs. These authors also noted a high frequency of associated pleural thickening in the costophrenic angles and in the interlobar fissures. Reduction of lung volumes, indicated by diaphragmatic elevation, is also a common manifestation of early bleomycin toxicity. Progressive toxicity may result in more homogeneous pulmonary opacities and lead to irreversible pulmonary fibrosis. CT is useful for evaluating patients with clinical findings of early bleomycin toxicity who have normal chest radiographs.12.26.27.38. and 194. CT often shows findings when the chest radiograph is normal. 12 In this setting, new linear or ground-glass opacities in a basal and frequently subpleural distribution suggest the diagnosis (Fig. 9.31). [18F]-Fluoro-deoxyglucose (FDG)-positron emission tomography (PET) imaging has been used to assess bleomycin toxicity.195. and 196.
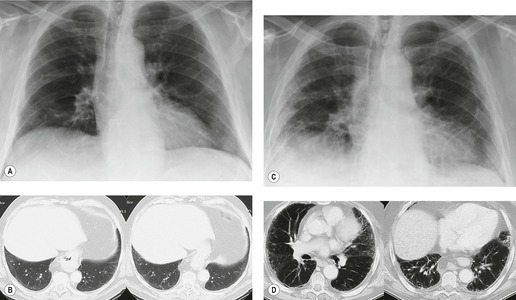 |
| Fig. 9.32 (Reprinted from Rossi SE, Erasmus JJ, McAdams HP, et al. Pulmonary drug toxicity: radiologic and pathologic manifestations. RadioGraphics 2000;20:1245–1259. Copyright Radiological Society of North America.) |
On occasion, discrete pulmonary nodules simulating metastases are seen on chest radiographs or CT.197.198. and 199. These nodules are usually peripheral in location, 5 mm to 3.0 cm in diameter and either sharply or poorly marginated (Fig. 9.34).27.194.197.199.200.201.202. and 203. In most cases, these lesions can be clinically differentiated from pulmonary metastases on the basis of appearance of the lesions (usually have more ill-defined margins than typical metastases), their relationship to bleomycin treatment, and the overall clinical scenario (lesions may appear while known tumor masses are decreasing in size). However, on occasion, confident differentiation of nodular bleomycin toxicity from metastases may require lung biopsy.
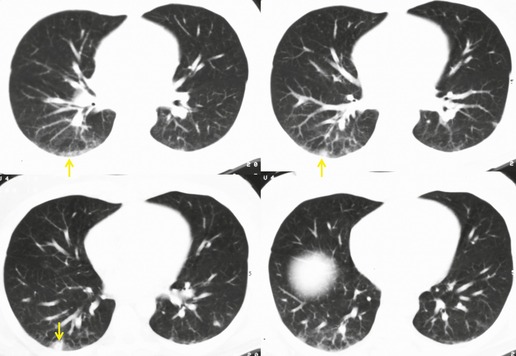 |
| Fig. 9.34 (Reprinted from Rossi SE, Erasmus JJ, McAdams HP, et al. Pulmonary drug toxicity: radiologic and pathologic manifestations. RadioGraphics. 2000;20:1245–1259. Copyright Radiological Society of North America.) |
Acute hypersensitivity to bleomycin manifests with rapidly appearing scattered pulmonary opacities that mimic pulmonary edema. These findings usually resolve quickly when therapy is discontinued.
Bromocriptine
Bromocriptine, an ergot alkaloid derivative, is an extremely effective and valuable treatment for Parkinson disease and prolactinemia. The chemical structure of bromocriptine is similar to that of methysergide, which may account for the fact that pleural effusions and pleural fibrosis occur in a small minority of patients receiving bromocriptine. Less than 5% of treated patients are affected and all reported patients have been men.204. and 205. Adverse effects appear to be confined to the pleura and adjacent lung. Symptoms and radiographic signs of effusion and pleural fibrosis usually take months or years to evolve. Slow resolution occurs after cessation of treatment, although it appears that the situation will stabilize if continuation of treatment is necessary.
Busulfan
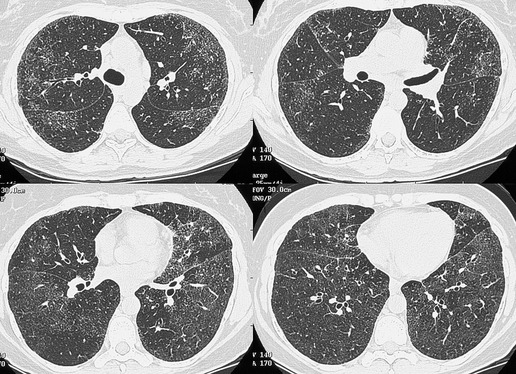
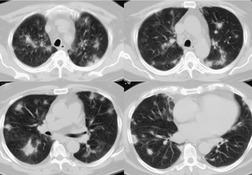
Busulfan, one of the earliest chemotherapeutic agents, is still used for treatment of chronic myelogenous leukemia. 206 The reported frequency of pulmonary toxicity ranges from 2% to 10% and typically occurs in patients who have received a total dose of more than 500 mg.27.194. and 200. Lung injury can, however, occur at low doses if the patient has had prior cytotoxic therapy.27. and 207. Evidence of pulmonary toxicity may be detected within months of starting treatment, but some patients do not show signs or symptoms of toxicity for several years. This variability makes the existence of a true dose–response relationship uncertain. Diffuse alveolar damage seems to be the most common histopathologic manifestation of busulfan-induced lung disease (Fig. 9.35).26.208. and 209. Interstitial pneumonia is less common and typically occurs only after prolonged administration of high doses of busulfan.26. and 210.
Cyclophosphamide (Box 9.10)
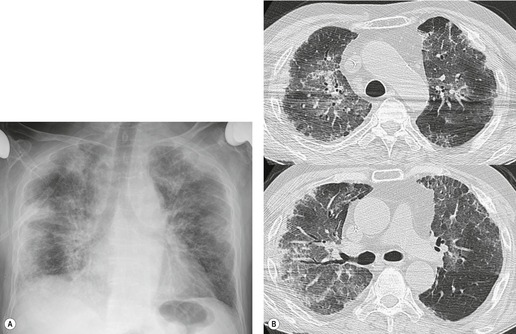
Cyclophosphamide is an alkylating agent used to treat a wide range of malignancies as well as benign conditions such as glomerulonephritis and Wegener granulomatosis. Diffuse alveolar damage is the most commonly reported histopathologic manifestation of cyclophosphamide-induced lung disease (Fig. 9.36, see also Fig. 9.4).26.29.211. and 212. IP and OP are less common (Fig. 9.37). 26 Despite its widespread use, cyclophosphamide-induced lung injury seems to be rare. 194 However, the fact that it is rarely used alone has made it difficult to gather data on its potential toxic side effects.
Box 9.10
• Commonly used for treatment of:
• Nonmalignant conditions: nephrotic syndrome, interstitial pneumonia, collagen vascular diseases, Wegener granulomatosis
• Malignancies: lymphoma, leukemia, breast and ovarian carcinoma
• Toxicity
• Occurs anytime from 2 weeks to 13 years after initiation of treatment
• Not obviously related to dose or duration
• Histopathology
• Diffuse alveolar damage most common
• Interstitial pneumonia, organizing pneumonia less frequent
• Radiology
• Nonspecific
• Basal reticulonodular opacities
• Pleural thickening can be a prominent feature
 |
| Fig. 9.36 (Reprinted from Rossi SE, Erasmus JJ, McAdams HP, et al. Pulmonary drug toxicity: radiologic and pathologic manifestations. RadioGraphics. 2000;20:1245–1259. Copyright Radiological Society of North America.) |
Careful analysis of a limited number of patients treated with cyclophosphamide alone suggests two distinct patterns of disease. 29 The first is an early onset pneumonitis that develops within 6 months of initiating therapy. Affected patients present with dyspnea, cough, and fever. 29 Chest radiographs show nonspecific basilar reticular or reticulonodular opacities. This patient group responds well to discontinuation of cyclophosphamide therapy and corticosteroid administration. The second pattern of disease is a chronic pneumonitis that occurs several months or years after prolonged treatment with cyclophosphamide. Clinical onset is insidious with progressive dyspnea and cough. 29 This form of toxicity has a much poorer prognosis, usually does not respond to corticosteroid treatment, and can lead to end-stage fibrosis (see Fig. 9.36). 29 Radiographic findings are nonspecific, but bilateral pleural thickening may be a prominent feature. 29
Cytosine arabinoside
Cytosine arabinoside (Ara-C) is an antimetabolite that inhibits DNA synthesis. It is effective in the treatment of acute leukemia in adults but can cause serious side effects depending on the total dose used. 213 Andersson et al. 213 reported a 22% frequency of subacute pulmonary toxicity occurring 2–21 days (median 6 days) after initiation of therapy. Toxicity primarily manifests as permeability pulmonary edema, although the precise mechanism of injury has not been established.214.215. and 216. The radiographic findings are those of pulmonary edema and include diffuse bilateral heterogeneous opacities that progress to diffuse homogeneous opacification217 (see Fig. 9.18). Small pleural effusions can be seen but are an insignificant feature. Following cessation of drug therapy and possible corticosteroid administration, chest radiographs may show clearing over a period of 1–3 weeks.
Diphenylhydantoin
The hydantoin derivatives such as diphenylhydantoin (Dilantin) are effective and widely prescribed antiseizure medications. Dilantin can cause diverse adverse reactions including gingival hyperplasia, hyperostosis, rashes, and lymphadenopathy.218.219.220. and 221. The most common site of lymphadenopathy is in the cervical region but other nodal groups including the hilar and mediastinal nodes may be involved (see Fig. 9.23). Clinically, Dilantin-induced lymphadenopathy has features of a hypersensitivity reaction. Affected patients typically present with maculopapular rash, hepatosplenomegaly, eosinophilia, and lymphadenopathy.219. and 220. The time of onset varies from 1 week to 30 years (median 5 years) after institution of therapy. 147 Biopsy of affected lymph nodes in these patients may show a spectrum of histopathologic patterns from reactive hyperplasia to immunoblastic hyperplasia. Progression to frank lymphoma, either Hodgkin or non-Hodgkin, occurs, but seems to be uncommon. 222
Fenfluramines
A group of anorectic drugs including fenfluramine and dexfenfluramine, and possibly phentermine, has been shown to cause pulmonary arterial hypertension and valvular heart disease.18.223. and 224. In patients taking these drugs for prolonged periods, the risk of pulmonary arterial hypertension is estimated to be 30 times that of the general population. 104 Not unexpectedly, these patients tend to be young women who are thereby exposed to risk of a potentially fatal disorder. Furthermore, these drugs have been shown to damage heart valves leading to valve incompetence. Valve injury is probably related to abnormal serotonin metabolism – a mechanism similar to that of the malignant carcinoid syndrome. However, unlike the malignant carcinoid syndrome, both right- and left-sided valves can be damaged. Some patients have required valve replacement or repair. 107
Gemcitabine
Gemcitabine is a nucleoside analog used to treat solid tumors such as nonsmall cell lung, pancreatic, and breast cancer that can cause significant lung toxicity (Fig. 9.38).12. and 25. Because it is usually given in combination with other agents, the precise frequency of toxicity is difficult to determine. Although dyspnea is reported in up to 10% of recipients,12. and 225. the best estimates suggest that serious toxicity is rare, affecting perhaps no more than 0.5% of treated individuals. 226 Numerous cases of fatal pneumonitis (presumably diffuse alveolar damage) caused by gemcitabine, either given alone225. and 227. or in combination with other agents (docetaxol,228. and 229. pacitaxel, 230 or vinorelbine230), have been reported.
Gold salts
Gold salts used in the treatment of rheumatoid arthritis probably cause pulmonary toxicity in about 1% of patients.48.190. and 231. Pulmonary toxicity is overshadowed by the far more frequent complications of stomatitis and dermatitis. Pulmonary toxicity occurs within 3 months of beginning of therapy and usually develops subacutely. The most common reaction is a hypersensitivity response. Chest radiographs show scattered or diffuse reticulonodular or more homogeneous pulmonary opacities (Fig. 9.39).231.232. and 233. Patients may have evidence of a hypersensitivity reaction in other organ systems, with fever, proteinuria, or a skin rash. Such manifestations of hypersensitivity may precede the pulmonary abnormalities and be associated with peripheral eosinophilia. Diffuse alveolar damage leading to fibrosis is also reported, but is much less common. Lupus caused by gold salts is rare. The major differential diagnostic problem is that the underlying disease, rheumatoid arthritis, can also cause lung disease. However, pulmonary opacities due to gold salts resolve in most instances after cessation of therapy, particularly if corticosteroids are administered. Pulmonary disease related to gold therapy also tends to be more acute in onset, whereas pulmonary involvement in rheumatoid arthritis is more insidious in onset and tends to be progressive.
Hydrochlorothiazide
Adverse reactions to the widely used diuretic hydrochlorothiazide are extremely rare. Nevertheless, the acute onset of a form of pulmonary edema within hours of taking the drug is well described. 234 Because hydrochlorothiazide is typically used to treat hypertension and heart disease, which can also cause pulmonary edema, the presence of this adverse drug reaction may not be recognized until readministration elicits the same reaction.
Interferons
Interleukin-2
Interleukin-2 is used in the treatment of disseminated malignancies, notably renal cell carcinoma and malignant melanoma. Interleukin-2 can cause a diffuse capillary leak syndrome due to toxic effects on the vascular endothelium. 235 Direct toxic effects on the myocardium236 can also contribute to edema, but pulmonary capillary wedge pressure usually remains normal. 237 Consequent depletion of intravascular volume can result in a clinical state analogous to the toxic shock syndrome, with diminished peripheral vascular resistance, hypotension, severe tachycardia, and decreased cardiac output.
The reported prevalence of interleukin-2 pulmonary toxicity varies. Radiographic abnormalities occur in 50–80% of treated patients.238.239.240. and 241. However, White et al. 242 found that only 4% of almost 200 patients undergoing treatment with interleukin-2 had clinical or physiologic evidence of significant respiratory compromise. They also found that the pulmonary side effects, which included diffuse edema and hypoxia, reversed rapidly with cessation of therapy.
The onset of signs and symptoms of toxicity is usually closely related to the initiation of therapy. In the series of Saxon et al., 241 radiographic abnormalities developed within 4 days in 50% of patients, within 8 days in 75%, and beyond 8 days in only 25%. The radiographic findings are essentially those of diffuse pulmonary edema (Fig. 9.40). Saxon et al. 241 described focal lobar or segmental consolidation in about a quarter of their patients but attributed these findings to nosocomial infection rather than interleukin-2 toxicity, an association also noted by Snydman et al. 243 Pleural effusions occur in over 50% of treated patients. The signs and symptoms of interleukin-2 toxicity usually resolve completely within 2 weeks after cessation of therapy. 244 Although the frequency of toxicity may be high, deaths attributable solely to interleukin-2 therapy are rare.
Methotrexate (Box 9.11)
Methotrexate, a folic acid analogue, is used extensively in the treatment of hematologic and other malignancies and is also used to treat a number of benign conditions such as rheumatoid arthritis and psoriasis. According to the most reliable estimate, pulmonary toxicity occurs in 7% of treated patients245 and is not related to duration of therapy or total cumulative dose. 27 The frequency of toxicity depends on the condition being treated. Patients with acute lymphoid leukemia have higher frequency of toxicity than patients with trophoblastic tumors or osteogenic sarcoma. 246 A 14% frequency of toxicity has been reported in patients with primary biliary cirrhosis. 247 However, patients treated with low-dose methotrexate for rheumatoid arthritis seem to have a much lower, if nonexistent, frequency of pulmonary toxicity. 248
Box 9.11
• Commonly used for treatment of
• Nonmalignant conditions: collagen vascular disease, interstitial pneumonitis
• Malignancies: lymphoma, leukemia, and breast carcinoma
• Toxicity
• Uncommon
• Idiosyncratic
• Not clearly related to dose or duration
• Histopathology
• Interstitial pneumonia most common
• Organizing pneumonia, diffuse alveolar damage less common
• Acute hypersensitivity is a rare manifestation
• Radiology
• Variable, nonspecific
• Hilar lymphadenopathy in 10%
• Basal reticulonodular opacities
The histopathologic, clinical, and radiographic and imaging manifestations of methotrexate toxicity are variable. Interstitial pneumonia is the most common histopathologic manifestation of methotrexate toxicity; organizing pneumonia and diffuse alveolar damage are less common (Figs 9.41 and 9.42).249. and 250. Fibrosis occurs in up to 10% of patients with methotrexate-induced pulmonary toxicity. A hypersensitivity response accompanied by pulmonary opacities and peripheral eosinophilia is also fairly common. 12




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree