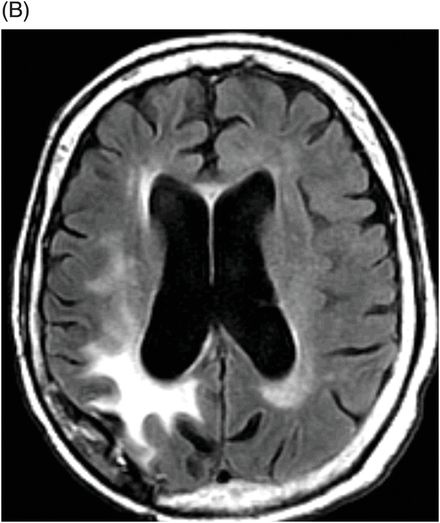
(A) Axial postcontrast T1WI through the ventricles and (B) Axial FLAIR image through the ventricles (six months after initiation of bevacizumab therapy).
Distal Glioblastoma Recurrence after Antiangiogenic Therapy
Primary Diagnosis
Distal glioblastoma recurrence after antiangiogenic therapy
Differential Diagnoses
Metachronous glioblastoma (GBM)
Metastasis
Imaging Findings
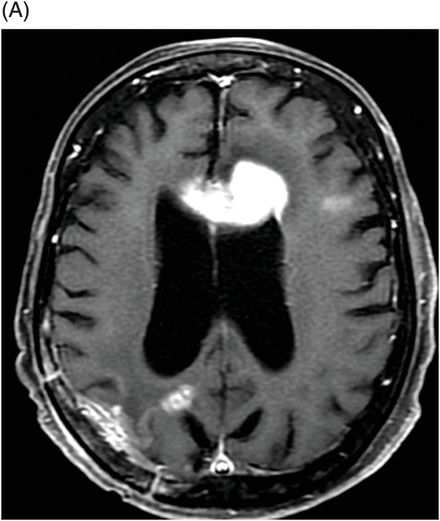
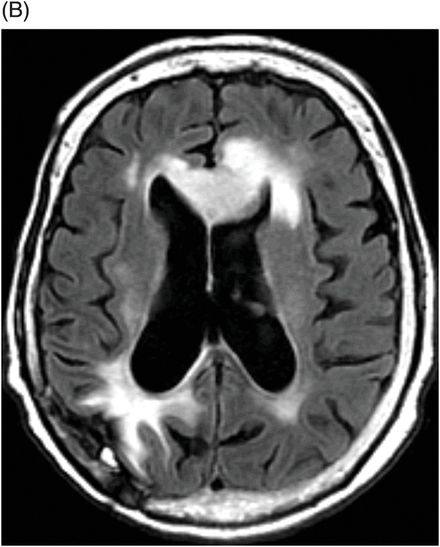
Fig. 95.1: (A) Axial postcontrast T1WI through the ventricles obtained four months after initiating bevacizumab therapy demonstrated subtle abnormal enhancement at the resection bed in the right parietal lobe without any nodular enhancing focus. No abnormal enhancement is noted in the genu of the corpus callosum. (B) Axial FLAIR image through the same level demonstrated FLAIR hyperintense areas in the right parietal lobe and in the periventricular white matter. There is no mass effect to the ventricle. No obvious FLAIR abnormality was noted in the genu of the corpus callosum. Fig. 95.2: (A) Axial postcontrast T1WI through the ventricles obtained six months after initiating bevacizumab therapy demonstrated intense enhancement of the left side of the genu of the corpus callosum, far away from the primary tumor site. Another subtle enhancement can also be noted at the subcortical left frontal lobe. (B) FLAIR image through the same level demonstrates new expansion and hyperintensity of the genu of the corpus callosum with mass effect to the left frontal horn. It can be noted that there is no significant change in the primary tumor site on both postcontrast as well as FLAIR images. There was also increased rCBV and diffusion restriction at the left side of the genu of the corpus callosum (not shown).
Discussion
New area of abnormal tumor-like enhancement distant from the primary tumor site in patients with known diagnosis of GBM and long history of treatment with bevacizumab is worrisome for distal GBM recurrence. Metachronous GBM is theoretically possible but is extremely uncommon outside the setting of bevacizumab therapy. The patient had no known cancer other than GBM, ruling out the possibility of brain metastasis from systemic cancer. Additionally, imaging features are not suggestive of metastasis.
In GBM, bevacizumab has been shown to induce dramatic improvement in tumor contrast enhancement as well as improvement of the peritumoral FLAIR abnormality and mass effect. Quality of life is significantly improved and time to progression is increased when bevacizumab is administered either alone or in combination with chemotherapy. While the initial response rate to bevacizumab in GBM patients is quite remarkable, it does not have any survival benefit. Additionally, some tumors do not respond to bevacizumab at all, and some tumors demonstrate rapid development of bevacizumab resistance. Although the underlying mechanism is not clearly understood, there are several explanations for the development of resistance to bevacizumab. The first hypothesis posits that evasive resistance to bevacizumab develops by recruiting alternative proangiogenic signaling, protecting the tumor vasculature with recruitment of proangiogenic inflammatory cells, or by increasing protective pericyte coverage and accentuated invasiveness of the tumor cells towards a more favorable microenvironment – either to co-opt a normal blood vessel or to metastasize to a distant place. The second hypothesis suggests that intrinsic resistance to bevacizumab therapy develops. The receptor tyrosine kinase, MET, a cellular receptor for hepatocyte growth factor, has recently been identified as a key mediator of tumor invasion, following angiogenesis inhibition. Several other molecular mechanisms have been identified that promote resistance to antiangiogenic therapy. In addition, antiangiogenic therapy activates GBM stem cells and can cause an angiogenesis-independent environment of tumor growth accompanied by the upregulation of proinvasive genes, dedifferentiation into endothelial cells, and acquisition of a more mesenchymal phenotype that favors invasive growth.
The incidence of invasion following bevacizumab therapy has been debated and its variability is mostly due to the lack of a universal definition of recurrence. Although GBMs treated with standard concurrent temozolomide and radiation therapy followed by adjuvant temozolomide demonstrate an increased incidence of recurrent and progressive disease outside of the defined contrast enhancement in up to 20% of patients, the incidence of distal recurrence outside of the defined contrast enhancement in patients treated with bevacizumab has been shown to be as high as 30–60%. This high incidence of distal recurrence in GBM patients with bevacizumab treatment has great clinical importance. Careful evaluation of the entire brain is essential for early identification of distal recurrence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree