Chapter 4 A Multidisciplinary Approach to Cancer
A Radiation Oncologist’s View
For many disease sites, the combination of radiation and chemotherapy has also been demonstrated to improve local disease control, enhance the effectiveness of organ-sparing approaches, and improve the curative potential of local treatments, presumably by sterilizing micrometastatic disease that would otherwise lead to the appearance of distant metastases. Randomized trials have proved that addition of concurrent chemotherapy to radiation improves local control and survival in patients with cervical, head and neck, lung, gastrointestinal, and other types of cancer. The use of chemoradiotherapy in the treatment of anal cancer has dramatically reduced the need for radical resection with colostomy for this disease.1–4
Radiation Biology
Another type of radiation-induced cell death is referred to as apoptosis or programmed cell death. Apoptosis can occur before or after mitosis.5 The plasma membrane and nuclear DNA may be important targets for apoptotic injury. Apoptosis appears to play an important role in the radiation response of some tumors and in certain normal tissues such as salivary glands and lymphocytes.
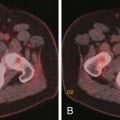
In vitro studies of the relationship between radiation dose and cell survival demonstrate that mammalian cells differ widely in their inherent radiosensitivity. These differences contribute to the wide range of doses required to cure tumors of different cell types. Even bulky lymphomas can typically be controlled with doses of 35 to 40 Gy, whereas 2- to 3-cm squamous carcinomas usually require doses of more than 60 Gy. Melanomas and most sarcomas require even higher doses and usually cannot be controlled with tolerable radiation doses if there is more than microscopic residual disease after surgery.
A number of factors influence cellular radiosensitivity and tumor responsiveness:
• Repair capacity: Cells differ in their ability to accumulate and repair sublethal injury. In general, normal tissues have a greater repair capacity than tumors. It is because of this difference that tumors can be controlled without unacceptable damage to irradiated normal tissues. However, some normal cells and tissues—particularly those that are rapidly proliferating, such as bone marrow and intestinal crypt cells—have relatively little repair capacity, and some tumors, such as prostate cancer, are able to accumulate and repair damage as effectively as most normal tissues. These variations influence the approaches used to treat various tumor types and sites.
• Although cells may also differ in their rate of repair, two-dose experiments and clinical experience suggest that most repair is accomplished within 4 to 6 hours. For this reason, schedules that involve more than one daily fraction of radiation are usually designed to require a minimum interfraction interval of approximately 6 hours to maximize repair of sublethal injury to normal tissues.
• Cell-cycle distribution: Cells are usually most sensitive during mitosis and in the late G2 phase of the cell cycle and most resistant in the mid- to late S and early G1 phases. The cell-cycle redistribution of cells after a dose of radiation may influence their overall sensitivity to a second dose.
• Hypoxia and reoxygenation: The dose of sparsely ionizing radiation required to effect a given level of cell killing is about three times greater under anoxic conditions than under fully oxygenated conditions. Although regions of hypoxia are present in many solid tumors and may have a role in the response to therapy, the clinical importance of hypoxia is diminished by reoxygenation that occurs as initially hypoxic cells become better oxygenated during a course of fractionated radiation therapy.6 To reduce the potential influence of hypoxia, radiation oncologists try to maintain patients’ hemoglobin levels at 10 to 12 g/dL or greater.
• Repopulation: The effect of cellular proliferation that occurs during a course of radiation therapy depends on the doubling time of the neoplastic cells and the total duration of treatment. Although the acute side effects of radiation usually limit the weekly dose of radiation to 900 to 1000 cGy/wk, many studies demonstrate that unnecessary protraction compromises local control and must be compensated for by increasing the dose of radiation.7–9 In addition, evidence suggests that radiation therapy as well as other cytotoxic treatment and even surgery can induce accelerated repopulation, increasing the detrimental effects of treatment protraction. Prolonged delays between surgical resection and initiation of radiation therapy may significantly compromise the efficacy of adjuvant radiation therapy.
Normal Tissue Effects of Radiation
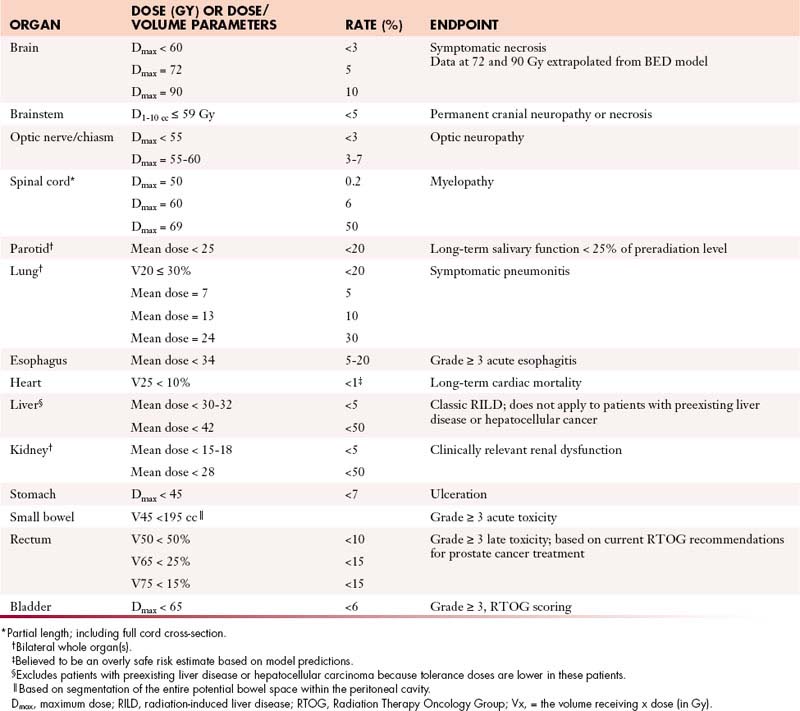
Tissues that are more slowly proliferating are referred to as late-responding tissues and tend to manifest side effects weeks or months after radiation therapy. These effects may reflect direct damage to parenchymal cells or damage to vascular stroma, and the dose-response relationship varies according to the tissue irradiated and other factors. Table 4-1 presents some of the conclusions of a 1991 task force10 charged with summarizing relevant data concerning the effect of ionizing radiation on normal tissues. A more detailed update was subsequently published in 2010.11 The duration of a course of radiation therapy has little impact on the incidence of late complications, but the dose per fraction has a major impact. In general, radiation schedules that involve fractional doses of 2 Gy or less permit maximal recovery of sublethal damage to normal tissues. For this reason and because acute side effects usually limit the weekly dose of radiation to no more than approximately 10 Gy, radiation therapy is most commonly delivered with a schedule of 1.8 to 2 Gy per fraction, five times per week. Most tumors repair cellular damage less effectively than late-responding normal tissues; as a result, the differential effect on tumor versus normal tissues is increased when a dose of radiation is fractionated. This is referred to as the fractionation effect.
Table 4-1 Approximate Dose/Volume/Outcome Data for Several Organs after Conventionally Fractionated Radiation Therapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree