Chapter 39 Complications in the Oncologic Patient
Chest
Chemotherapy-Induced Noninfectious Lung Disease
The problem of drugs adversely affecting the lungs remains a major challenge to all primary care physicians. Symptoms are usually nonspecific, with patients presenting with dyspnea, nonproductive cough, and fever, which can begin weeks to years after the medication is first taken. Unfortunately, drug-induced respiratory disease remains a disease of exclusion because the majority of drugs responsible for this effect cannot be diagnosed by any specific test. Diagnosis requires a high index of suspicion because infection, radiation pneumonitis, and recurrence of the underlying disease can manifest clinically and radiographically in a similar manner. There is underreporting of drug toxicity, and thus, the true incidence of drug-induced respiratory disease is unknown, although it is estimated to be less than 10%.1 To complicate matters, only a few patients are treated with single drugs. Thus, how much of an observed reaction is related to one agent or another versus synergy with other drugs, oxygen, or radiation often remains unknown. Prompt diagnosis of drug-induced lung toxicity is important because early drug-induced lung injury will often regress with the cessation of therapy or with initiation of steroid therapy, before the development of pulmonary fibrosis. For an updated list of generic drugs, type of reaction, and radiologic manifestations with references, the reader is referred to the routinely updated website www.pneumotox.com.2 In this reference and in Table 39-1, the reader will find some of the effects of drugs on other chest structures such as those causing pleural effusions, lymphadenopathy, or cardiomyopathy.
Table 39-1 Histologic Patterns Associated with Drug Reactions
| FINDING | DRUG |
|---|---|
| Noncardiogenic pulmonary edema | Carbamazepine, gemcitabine, taxanes, cyclophosphamide, methotrexate, vinblastine |
| Alveolar hemorrhage | Bevacizumab, rituximab |
| Alveolar proteinosis–like reaction | Mitomycin C |
| Diffuse alveolar hemorrhage | Carbamazepine, methotrexate, gemcitabine |
| Diffuse alveolar damage | Gemcitabine, methotrexate, bleomycin, cyclophosphamide, gefitinib, erlotinib |
| Organizing pneumonia | Bleomycin, cyclophosphamide |
| Bronchiolitis obliterans syndrome | Busulfan |
| Usual interstitial pneumonia–like pattern | Bleomycin, gemcitabine, methotrexate, busulfan, cyclophosphamide |
| Diffuse cellular interstitial infiltrates with or without granulomas | Bleomycin, methotrexate |
| Nonspecific interstitial pneumonia | Bleomycin, taxanes, gemcitabine, methotrexate, imatinib |
| Desquamative interstitial pneumonia | Busulfan |
| Acute or chronic eosinophilic pneumonia | Bleomycin, methotrexate |
| Pulmonary veno-occlusive disease | Bleomycin, busulfan |
| Pulmonary nodules | Bleomycin, vinblastine |
| Pneumothorax/pneumomediastinum | Bleomycin |
| Hilar/mediastinal adenopathy | Bleomycin |
| Pleural effusion | Methotrexate, cyclophosphamide, imatinib |
| Cavitations | Bevacizumab |
| Pulmonary thromboembolism | Bevacizumab, thalidomide |
| Cardiomyopathy80 | Doxorubicin, 5-fluorouracil, trastuzumab |
Modified from www.pneumotox.com; and Flieder DB, Travis WD. Pathologic characteristics of drug-induced lung disease. Clin Chest Med. 2004;25:37-45.
The lungs have a limited number of histopathologic responses to injury (see Table 39-1) that mimic many other pulmonary conditions.1 The radiologic appearance of drug toxicity corresponds to the histopathologic finding. Owing to these nonspecific findings, diagnosis rests on correlation with clinical, laboratory, and radiologic information.3 Even bronchoalveolar lavage (BAL) can only suggest an iatrogenic cause by helping to exclude infection and malignancy. Sometimes, cell composition in the lavage and elevation of a specific population (e.g., lymphocytes, neutrophils, or eosinophils) may suggest a more specific diagnosis.
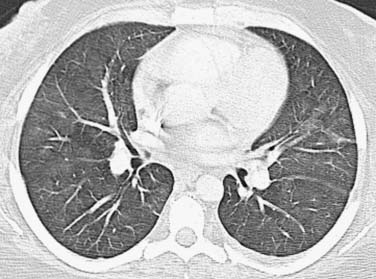
As an aid for interpretation of the chest images, it is helpful to divide drug toxicity into acute and delayed presentation. Acute-onset, chemotherapy-induced lung injury is usually caused by noncardiogenic pulmonary edema/diffuse alveolar damage, hypersensitivity-type reaction, or pulmonary hemorrhage and occurs after the initial dose of chemotherapy. Radiographic findings usually include diffuse or scattered ground-glass opacities (GGOs) or consolidative opacities with or without septal thickening (Figure 39-1).
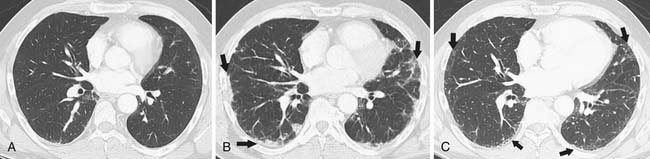
Delayed-onset, chemotherapy-induced lung injury usually presents more than 2 months after the completion of treatment or during prolonged treatment and is often caused by chronic interstitial pneumonia, which can lead to fibrosis. The more common histologic patterns seen are usual interstitial pneumonia (UIP) or nonspecific interstitial pneumonia (NSIP).3 The pattern most commonly encountered is that of NSIP, which appears as bilateral lower lobe predominant heterogeneous or consolidative opacities on chest radiographs, and on thin-section chest computed tomography (CT), it appears initially as scattered GGOs or consolidative opacities with lower lobe predominance (Figure 39-2). Although intralobular septal thickening and traction bronchiectasis can be seen with NSIP, when present, these findings are much more likely to be from UIP.4,5 Honeycombing is typically seen with UIP. Organizing pneumonia with fibromyxoid connective tissue plugs that fill distal airspaces as well as terminal or respiratory bronchioles is another nonacute form of drug toxicity. When associated with a known causative agent, this finding should not be termed “cryptogenic organizing pneumonia,” but rather identified by its older description: bronchiolitis obliterans organizing pneumonia (BOOP) caused by the name of the drug (e.g., BOOP caused by amiodarone).3–5 Radiographic findings of drug-induced BOOP on chest radiographs are bilateral scattered heterogeneous or consolidative opacities in a peripheral distribution, and on chest CT, there are unilateral or bilateral areas of consolidation, with either peripheral or the more typical peribronchovascular distribution and a lower lobe predominance.6 Rarely, BOOP may have a nodular appearance that can be confused with metastatic disease.7
Key Points Drug toxicity
Chemotherapy-Induced Infectious Lung Disease
Drugs that are used to combat cancer target dividing cells, affect the bone marrow, and cause a decrease in neutrophils resulting in infections. Other drugs often used to treat hematologic malignancies, in conjunction with chemotherapeutic agents, have immunosuppressive properties. Corticosteroids cause a broad suppression of the immune system, whereas the newer targeted monoclonal antibody therapies, such as rituximab, cause specific suppression.8 Diagnosis of pneumonia depends on clinical symptomatology with radiographic findings. The type of pathogen producing the pneumonia, whether bacterial, viral, or fungal, depends mainly on the type of host and combination therapy they received. Given the inability of the severely immunocompromised host (e.g., SCT recipients or prolonged neutropenia in patients with hematologic malignancies) to mount an adequate inflammatory response, the classic radiographic findings of each type of pneumonia may differ from those found in immunocompetent patients, and chest films may even appear normal. CT may disclose more subtle changes, such as minimal GGOs, bronchial thickening, or nodules, and in select cases, may show the typical appearance for a specific group of pathogens.9 Such findings may lead to the correct selection of antibiotic therapy and improved outcomes.10,11
Bacterial Pneumonia
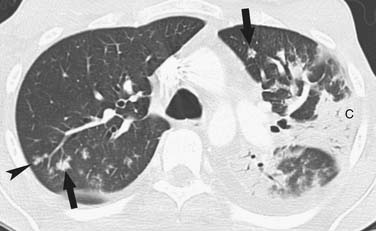
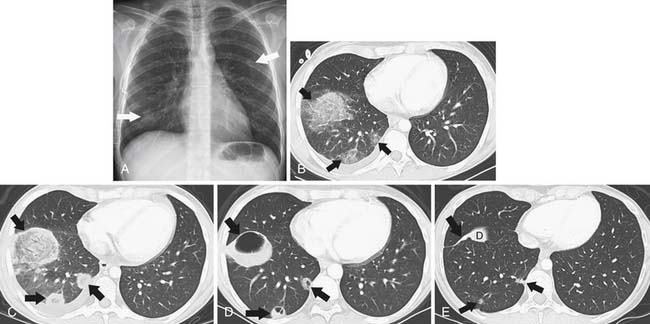
The main source of pathogens are from the patient’s endogenous flora.12 With the introduction of extended-spectrum beta-lactams, there has been a decrease in bloodstream infections due to gram-negative rod bacteremia and an increase in infections due to gram-positive cocci,13 although nosocomial bacterial pneumonias are still predominated by gram-negative rods.14 There is a low predictive yield to determination of the type of bacterial pneumonia from the chest radiographic or even chest CT appearance in the immunocompromised patient population. This is because of coexisting abnormalities such as edema, atelectasis, or aspiration and also because of the diminished immunologic response to infection as the result of therapy.15 In SCT recipients, bacterial pneumonia most commonly manifests as pulmonary nodules (81%), either large or small, in a tree-in-bud distribution, lower lobe predominant, asymmetrically distributed consolidation (69%), and GGOs (35%), usually symmetrically distributed.16 The majority of patients demonstrate a combination of these findings (73%), with another 15% of patients having pulmonary nodules only and 12% consolidation only on CT (Figure 39-3).
Viral Pneumonia
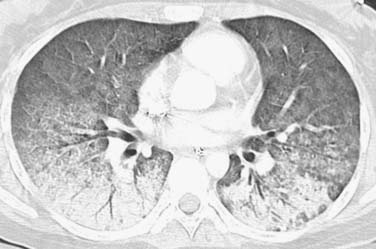
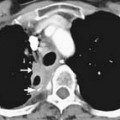
DNA viruses such as herpes simplex, varicella, and cytomegalovirus (CMV) have long been recognized as causing severe respiratory infections in patients with hematologic malignancies.17 These patients are also exposed to community seasonal respiratory viruses such as adenovirus or influenza A, which can be life-threatening in such immunocompromised patients.18 Radiographically, one cannot differentiate one viral infection from another. They tend to be symmetrically distributed in the lung, usually with lower lobe predominance and a combination of GGOs or consolidative airspace disease (Figure 39-4) in addition to small centrilobular nodules and consolidative opacities.18–22 Mortality rate can be high and early diagnosis is essential because early treatment improves survival. Of this group, airspace disease is the most common finding, seen in 90% of patients with CT-documented pneumonia.18,21,22 The early changes of lower lobe–predominant peribronchial thickening and peribronchial GGOs or tree-in-bud opacities are more readily appreciated on a lateral plain film or, with greater sensitivity, by chest CT, although early bacterial pneumonia may have a similar appearance.
Fungal Pneumonia
Opportunistic invasive fungal pneumonias are associated with high morbidity and mortality rates23–25 and are typically found in patients with prolonged, severe immunocompromise, as can be seen with hematologic malignancies and SCT recipients, but is rarely encountered in patients with solid malignancies. Although invasive pulmonary aspergillosis (IPA) and Candida are the most common, other angioinvasive molds, such as Fusarium and Zygomycetes species are increasingly encountered in these severely immunocompromised hosts. Because early institution of high-dose antifungal therapy is associated with improved outcomes,10,26 early recognition of invasive fungal disease is important. However, cultures of respiratory secretions are neither sensitive nor specific, and lavage and invasive procedures often cannot be done in these patients because of coagulation abnormalities and thrombocytopenia.27,28 Thus, diagnosis of invasive pulmonary fungal disease relies heavily on imaging.29 CT is often used in an attempt to identify fungal pneumonia in a timely fashion because some of these typical imaging findings cannot be detected by chest radiographs.
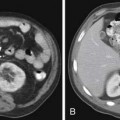
The “halo sign,” a nodule surrounded by GGOs, is seen in 92% of patients with IPA at presentation.10 The presence of large nodules (>1 cm) and visualization of the halo sign are most suggestive of fungal infection.16 There have been attempts to differentiate pulmonary zygomycosis (PZ) from IPA because this distinction has important therapeutic implications: specifically, voriconazole, the preferred drug in the treatment of IPA,30 has no activity in zygomycosis.2 The presence of the “reversed halo sign” (Figure 39-5), a focal round area of ground-glass attenuation surrounded by a ring of consolidation, or the presence of multiple nodules (>10) is more commonly seen with PZ than with other invasive fungi.31,32 Although these CT findings of the reversed halo sign and halo sign are not pathognomonic for fungal infections and can be seen, for example, with cryptogenic organizing pneumonia, infarction, or malignancy, when observed on imaging of a febrile, severely immunocompromised patient, these should be considered invasive fungal pneumonia until proved otherwise.
Pneumocystis pneumonia (PCP), once a major cause of morbidity and mortality in patients treated for hematologic malignancies, has been largely prevented by the use of antimicrobial prophylaxis. Chest radiographs at presentation can be normal and later nonspecific with bilateral, perihilar reticular and poorly defined GGOs, which often progress to alveolar consolidation in 3 to 4 days.33 Thin-section CT usually reveals scattered ground-glass attenuation that can be associated with interlobular septal thickening, findings that can resemble viral pneumonia such as CMV pneumonia. In an attempt to differentiate the CT appearance of CMV from PCP in non–human immunodeficiency virus (HIV) patients, apical distribution and a sharply demarcated mosaic pattern are found more frequently in PCP, whereas small nodules or unsharp demarcation of GGOs and lower lobe predominance are more likely to be seen in CMV pneumonia.34
Tuberculosis
Although patients immunosuppressed from chemotherapy are at risk for tuberculosis (TB), the incidence of tuberculosis in even the most severely immunosuppressed, such as SCT recipients, is proportional to the incidence of TB in the general population. The imaging appearance of TB in this population is identical to that in the general population.35,36
Key Points Chemotherapy-induced pneumonia in the severely immunocompromised host
• Bacterial pneumonia most commonly manifests as a combination of consolidation/GGOs with tree-in-bud lower lobe–predominant nodules.
• Viral pneumonia most commonly manifests as diffuse lower lobe–predominant consolidation/GGOs with or without centrilobular nodules.
• The presence of large nodules (>1 cm) and visualization of the halo sign are most suggestive of fungal infection.