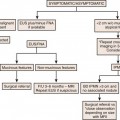
Chapter 38 Interventional Imaging in the Oncologic Patient
Image-Guided Tissue Ablation
Technical Background
Radiofrequency Ablation
Image-guided percutaneous RFA is typically guided by computed tomography (CT), ultrasound (US), or a combination of both. RFA is performed by connecting a generator that provides an electric to a metallic applicator probe. Thermal energy is applied onto tissues through the tip of the probe. The tissues surrounding the tip are destroyed within seconds as temperatures reach 55°C and destroyed immediately at temperatures greater than 60°C. Care is taken to avoid charring tissues, which limits heat propagation. During RFA, an alternating electrical current (frequency range 480-500 kHz) is deposited within the tissues via an electrode placed directly into the tumor. The resulting ionic agitation within the lesion and surrounding tissues generates heat in the immediate vicinity of the electrode. The heat is then conducted to the surrounding environment, resulting in ablation of a finite volume of tissue. Ideally, the ablation zone should encompass the tumor and a 5- to 10-mm margin of normal tissue. Thus, the periphery of the ablation zone consists of a rim of hyperemia and inflammatory reaction involving the surrounding liver parenchyma. The size and shape of the ablation zone will vary depending on the amount of energy, type of electrode, duration of ablation, and inherent tissue characteristics.1 Image-guided RFA has been used to treat tumors in a wide variety of organs such as liver, kidneys, lung, and bone, among many others. Initial RFA indications included the treatment of small lesions in patients who were not surgical candidates or for palliation of large lesions. However, owing to the efficacy and safety profile of the technique, its use has greatly expanded in certain diseases that now also include patients who are surgical candidates with comparable outcomes.2 The limitations of the technique include the “heat-sink effect,” in which adjacent blood vessels larger than 3 mm lead to perfusion-mediated attenuation of thermal energy deposition, potentially leading to incomplete ablation; large (>5 cm) complex lesions; and proximity to delicate structures, such as gastrointestinal wall, gallbladder, diaphragm, nerves; among other effects.
Cryoablation
Cryoablation is typically guided by CT or magnetic resonance imaging (MRI). Cryoablation consists of the application of freezing temperatures onto tumors in order to cause tissue destruction. This technique has been used to treat tumors in a variety of tissues such as liver, kidney, prostate, lung, and cervix.3–5 In order to perform cryoablation, a metallic probe is directly inserted into the target lesion. Argon gas circulates through the probe, causing a rapid drop of the local temperature. The ensuing low temperatures cause disruption of the cellular membrane and local ischemia. Ice crystals form within the cells and the adjacent interstitium, causing cell dehydration and surrounding vascular thrombosis. Subsequently, when the tissues thaw, vascular occlusion leads to further ischemic injury.6 Consistent tumor cell death is accomplished when the tissues are exposed to temperatures of at least –20°C, corresponding to the area approximately 3 mm inside the margins of the ice ball. The temperatures along the interface between the ice ball and the adjacent tissues, as well as in the ice ball’s peripheral rim, are suboptimal for tissue necrosis and represent only a reference for the interventional oncologist. As with RFA, the main limitations of cryoablation include proximity to blood vessels, gastrointestinal organs, nerves, and skin. Treatment of large tumor volumes with cryoablation can lead to the development of important systemic complications, such as cryoshock, a cytokine-mediated inflammatory response associated with coagulopathy and multiorgan failure; myoglobinuria; and severe thrombocytopenia.7–9 Otherwise, most complications of cryotherapy—such as hemorrhage and injury to adjacent organs—are generally similar to those of RFA.
Clinical Applications
Liver Tumors
RFA is routinely performed worldwide for the curative or palliative treatment of liver tumors. There is ample literature documenting the successful use of minimally invasive RFA for both resectable and nonresectable liver lesions. Hepatocellular carcinoma (HCC) and metastatic colorectal carcinoma are the most common malignancies that affect the liver. The literature supports the use of percutaneous RFA in patients with HCC and either a single lesion smaller than 5 cm in diameter or up to three lesions smaller than 3 cm in diameter, if partial hepatic resection or transplantation is not available.10 Recent extensive review of the literature regarding RFA in the treatment of colorectal liver metastasis by Mulier and coworkers11 indicates a similar rate of local recurrences after open RFA versus surgical resection for colorectal liver metastasis smaller than 3 cm. In addition, RFA can be used to treat a wide variety of other unresectable liver tumors or theoretically resectable lesions in patients who are not surgical candidates.
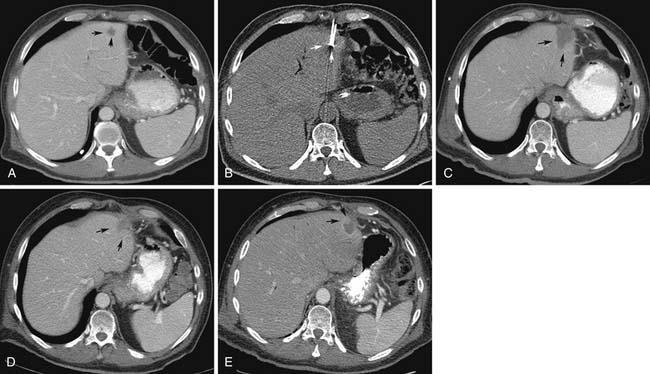
During the initial postprocedure follow-up imaging, the completeness of the ablation has to be carefully scrutinized. Dual-phased imaging is recommended to optimally characterize postintervention findings. The early arterial phase is essential for hypervascular lesions, such as HCC and neuroendocrine tumors. The portal venous phase is ideal for all other lesions and the combination increases the sensitivity of the study. The zone of ablation can be readily identified as an area showing lack of contrast enhancement and overlapping the location of the original lesion. This area has to have least 0.5 cm of its margins extending beyond the original confines of the target lesion on all sides. If this safety margin is not present, the radiologist needs to have a high index of suspicion for residual disease. When CT is the modality used for guidance, a contrast-enhanced study can be performed at the end of the treatment and any suspicious areas subjected to additional ablation. The presence of a uniform, rather than a nodular, rim of enhancement around the ablated area shortly after the procedure is usually benign and represents inflammatory reaction.12–15 Likewise, a central area of increased attenuation on CT scans immediately after ablation is usually benign in nature.12,13 Other expected findings commonly seen shortly after ablation that should not raise concern for complications include portal venous gas and air bubbles within the treatment cavity. Over time, the adequately ablated lesion becomes well-circumscribed and shows gradual size involution. Any areas showing enlarging nodular peripheral enhancement are concerning for residual or recurrent disease (Figure 38-1). A review of comparison prior studies is usually sufficient to establish the diagnosis. However, when an area is deemed questionable, but not definite, for recurrent or residual disease, a logical next diagnostic step is to proceed with fluoro-2-deoxy-D-glucose (FDG)–positron-emission tomography (PET) or PET/CT, which have been shown to be more accurate than CT in this particular scenario.16,17
The most common complications after hepatic RFA include: postprocedural hemorrhage, liver abscess with an incidence of approximately 2%, bile duct injury with biloma formation, hepatic infarction, and injury to adjacent organs.18,19 Hemorrhage is readily identified in the immediate postprocedural CT scans as a hyperattenuating fluid collection on nonenhanced images. Abscess formation should be suspected when new gas bubbles in the treatment bed are identified that were not present on the initial post RFA scan. Focal dilatation of bile ducts peripheral to the ablation cavity is commonly observed, but usually requires no further intervention. Along the same lines, development of a low-attenuation fluid-filled cavity within the parenchyma is suggestive of biloma formation. Bilomas tend to have a benign course, rarely necessitating drainage.
Renal Tumors
The widespread use of US, CT, and MRI for the investigation of abdominal pathologies has led to the diagnosis of a large number of incidental renal tumors. Renal nodules displaying postcontrast enhancement of cross-sectional images are assumed to be malignant until proved otherwise. Because these lesions are increasingly being discovered, when they are still small in size, therapeutic options that spare renal function are highly desirable. Minimally invasive percutaneous procedures offer a safe alternative to partial nephrectomies.20 The thermal ablation techniques typically employed in the treatment of such tumors, include RFA and cryoablation. The ideal lesion for RFA should be small in diameter (<3 cm) and peripheral in location. Central lesions are usually better treated with cryotherapy, owing to the decreased risk of ureteral stricture. A meta-analysis of 47 studies that compared cryoablation with RFA found that repeat ablation was required more frequently with RFA (8.5% of cases vs. 1.3% for cryoablation) and that local tumor progression occurred more frequently with RFA (12.9% vs. 5.2%).21 Metastatic disease was also more common in the RFA group (2.5% of cases vs. 1%). One series comparing cryoablation with RFA found less tumor persistence or recurrence with cryoablation (11.1% of cases vs. 1.8%).22
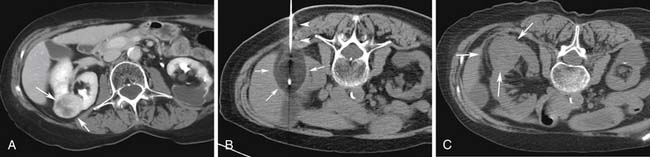
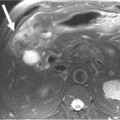
The radiologist has to be familiar with the different appearances of the zone of ablation in the treated kidney (Figure 38-2). In order to do so, it is imperative to understand the gradual evolution of the completed area, potential complications, and signs of residual or recurrent disease. The expected natural course of a successfully treated renal mass is to slowly decrease in size over 24 months, finally reaching approximately half of its original size. A caveat may be observed on the initial follow-up images after treatment of small lesions when a slight increase in lesional volume is occasionally noted. This finding may be secondary to ablation of normal adjacent renal parenchyma. The typical zone of ablation does not show contrast enhancement. A focal area of enhancement should raise suspicion for residual or recurrent disease, particularly if nodular in morphology. During the precontrast phase, the ablation bed tends to be hyperattenuating compared with the adjacent renal parenchyma on CT scans. This feature indicates the presence of proteinaceous material in the treated area. Similarly, these products lead to increased signal on the nonenhanced MRI T1-weighted sequences. Particular to MRI is a thin rim of peripheral enhancement due to the presence of postablation inflammatory changes. This finding is usually not identified on CT scans, probably owing to the lower signal-to-noise ratio.
Lung Tumors
CT and PET scans are the imaging modalities most commonly employed to image lung lesions after ablation. Follow-up imaging is usually performed approximately 1 month after the initial treatment, subsequently at 3- to 6-month intervals up to 24 months. Typically, the ablation zone is identified on follow-up CT scans as a nonenhancing hypoattenuating lesion, whereas areas of tumor progression tend to exhibit enhancement greater than 15 Hounsfield units (HU). A rim of peripheral enhancement on the initial posttreatment images is usually secondary to the inflammatory reaction around the ablation cavity rather than residual or recurrent disease. In addition, ground-glass opacities may be observed around the zone of ablation in the immediate postprocedural scans.23 Owing to these early postablative changes surrounding the target lesion, interpretation of initial posttreatment CT scans may be difficult in cases in which the target lesion appears larger in size but no obvious areas of enhancement are visualized. In this particular scenario, the use of FDG-PET/CT is useful to help distinguish areas of residual viable tumor cells. Although initial inflammatory changes around the zone of ablation may lead to early false-positive results, the presence of areas of showing increased FDG uptake after 3 months should be considered suspicious for residual disease.24
The most common complications of lung ablation include pleural effusion, pneumothorax, bronchopleural fistula, hemoptysis, infection, and potential worsening of the underlying lung disease. The incidence of pneumothorax revolves approximately 28% with approximately 10% of patients requiring chest tube insertion.25
The estimated stage I lung cancer survival rates are 70%, 57%, 36%, 27%, and 27% for 1, 2, 3, 4, and 5 years, respectively, after initial lung RFA. These numbers compare favorably with those from a recent study investigating external beam radiation therapy alone for patients with inoperable stages I to II non–small cell lung cancer.25