Chapter 2 A Multidisciplinary Approach to Cancer
A Surgeon’s View
Introduction
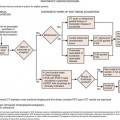
Surgery enables long-term survival and remains the modality central to the opportunity for cure in patients with solid tumors. In order for surgery to be effective, patients must be selected properly so that nontherapeutic surgery is avoided. In the current era of cancer surgery and imaging, “exploratory surgery” should, with the rarest exceptions, not exist as a diagnostic modality. Resectability rates are rising based on improved imaging, but they are admittedly not perfect yet. Whereas involvement of the superior mesenteric artery is predicted with virtually 100% accuracy in pancreatic adenocarcinoma, there are some tumor-vessel relationships that cannot be defined with 100% radiologic-clinical correlative accuracy (e.g., hilar cholangiocarcinoma abutment or involvement of a sectoral hepatic artery) and small volume peritoneal disease may not be detected on even the best preoperative imaging.1,2 Furthermore, the interaction between different treatments, including chemotherapy with and without biologically active agents, radiotherapy, intra-arterial therapies, and surgery, require that treatment sequencing, timing, and duration of therapies be considered carefully and contribute to the constant movement in the line defining resectability for many tumors. Before patients embark on complex treatment plans, treatment sequence/timing issues must be considered by a team of physicians—similarly before a patient is declared unresectable (e.g., before applying frequently noncurative therapies such as ablation for hepatic tumors), the multidisciplinary team must often weigh in to assess the spectrum of treatment options. Open communication between surgeons and radiologists change the way surgeons operate and change the way radiologists report their findings to optimize patient care. Patient care is just that—patient care. If the focus of the radiologist, surgeon, radiotherapist, oncologist, and others in the care team is constantly on the patient, the best outcomes can be achieved. If the surgeon operates, the oncologist gives chemotherapy, and the radiation oncologist delivers radiotherapy based on a piece of paper (e.g., a radiology report), the best care cannot be delivered. If the radiologist is integrated into the treatment team, modern, rapidly improving patient outcomes realized in centers of excellence can be achieved more widely. Finally, goals of care differ in different patients. In some, the goal is prevention, in others, diagnosis and treatment. In yet others, the goal may be palliation. Achieving these goals requires actual integration of the members of the treatment team to work together in a patient-focused way.
Candidacy for “potentially curative” therapy is rapidly changing. As an example, many physicians and patients are not aware that multiple, bilateral liver metastases from colorectal or neuroendocrine primary cancers can be treated with curative intent, leading to survival rates exceeding 50% at 5 years post resection.3–5 In such cases, radiology reports of “multiple, bilateral liver metastases” may be accurate, but they may also be misleading to the patient or even to the oncologist/gastroenterologist reading the report (discussed later). Sadly, many clinicians cannot or do not read their films at the time they read the radiology report, and they may misinterpret findings. These factors contribute to the need for precise communication among members of the care team to optimize the value of imaging and optimize patient care. This chapter outlines the following areas:
Diagnosis
In many cases, however, pathologic diagnosis is needed, either to confirm the clinical/radiologic suspicion, as a requirement for treatment by radiotherapy or chemotherapy, or as a requirement for protocol-based therapy. In these cases, the initial imaging will often define whether a percutaneous or an endoscopic approach to biopsy is needed. When percutaneous biopsy is planned, the presumed tumor type and location affect biopsy planning. For liver tumors, biopsy technique significantly affects needle-tract seeding, which should be an extremely rare event (<1%).6,7 Ultrasound-guided lymph node biopsy with core biopsy can provide a diagnosis of lymphoma, although excisional biopsy may be needed and is guided by both clinical examination and cross-sectional imaging. More recently, FDG-PET may demonstrate the most active nodes and guide biopsy planning as well. Seeding is virtually unheard of with the introduction of endoscopic ultrasound–guided biopsy throughout the gastrointestinal tract. Thus, even at the level of obtaining diagnosis, consideration as to the probable diagnosis and possible treatments must often be given, reemphasizing the need for a multidisciplinary approach when treating cancer patients.
Surgical Planning
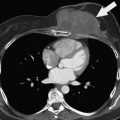
Two different examples are described here. First, in the case of pancreatic adenocarcinoma, whether in the head, body, or tail of the pancreas, vascular abutment, encasement, or occlusion by the tumor have been well defined and provoke significantly different treatment approaches (these are discussed in subsequent chapters, and summarized here).8 Further, the vessel involved is important—arterial versus mesenteric/portal venous involvement has significantly different surgical and oncologic implications. In the case of no vascular involvement, straightforward pancreatectomy is generally planned, with pre- or postoperative chemoradiotherapy. In the case of venous involvement, an entirely different operative plan is made to include vascular resection and reconstruction; this picture can change with neoadjuvant therapy. Venous abutment and encasement may not exclude resectability, whereas venous occlusion is considered “borderline” and may be resectable in selected cases. In the case of arterial involvement, preoperative therapy may be advised, and in the case of extensive (>180-degree encasement of the artery), surgery is simply not indicated.8 The finding of liver metastasis excludes the therapeutic value of surgery, even for the smallest resectable primary pancreatic adenocarcinoma. Regional adenopathy does not exclude resectability. Suggestion of peritoneal disease, which is not definitive, may prompt staging laparoscopy. Thus, accurate staging and reporting of findings relevant to surgery for the specific disease are critical to surgical planning and result from communication between imaging and treating physicians.
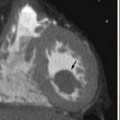
As a different example, issues in liver surgery can be even more complex. Resectable liver tumor(s) are often defined based on liver that will remain after resection, including preservation of adequate inflow and outflow to the preserved segments, with adequate liver remnant volumes.9,10 Tumor-vessel relationships within the liver affect resectability differently from that for pancreatic or other gastrointestinal, thoracic, head and neck, or extremity tumors. Tumors may involve two of three outflow vessels (hepatic veins) in the liver and abut the inferior vena cava but be resectable with standard techniques and excellent results.11 Rarely, involvement of all three hepatic veins, traditionally considered a sign of unresectablilty, is not an impediment to complete resection either because vascular resection/reconstruction can be considered or because venous anomalies may permit otherwise impossible resections such as subtotal hepatectomy based on the presence of a dominant inferior right hepatic vein.12,13 Major hepatectomy includes resection of tumors involving major hepatic and portal branches routinely, as long as vessels supplying and draining the liver remnant are free of tumor. In other cases, major resection is possible because of other anatomic variations in the liver, such as a staged portal bifurcation allowing resection of tumors involving the central liver.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree