Ferenc A. Jolesz (ed.)Intraoperative Imaging and Image-Guided Therapy201410.1007/978-1-4614-7657-3_34
© Springer Science+Business Media New York 2014
34. A Rationale for the Use and Development of Methods for Image-Guided Brain Tumor Surgery
(1)
National Center for Image Guided Therapy, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Abstract
For over 80 years, researchers and clinicians have acknowledged the difficulty of achieving optimal outcomes during brain tumor resection. In the past decade, a growing body of evidence has emerged linking extent of surgical resection to outcome. Nonetheless, safely maximizing extent of resection remains a central challenge in modern neurosurgical oncology. A variety of technologic innovations have been developed to assist surgeons in performing the best possible brain tumor resections. This chapter will focus on how current perspectives on glioma surgery justify the development and use of technologic innovations to ensure maximal safe resection.
Why More Is Better: Extent of Resection and Glioma Surgery
The impact of aggressive resection for gliomas on patient outcome has been an area of controversy since the beginning of the modern era of neurosurgery [1–3]. Given the typically benign natural history of low-grade gliomas, their infiltrative growth patterns, and the risk for permanent neurologic deficit associated with surgical intervention, the role of extensive resection has been questioned. Nonetheless, most studies have suggested gross total resection is associated with improved survival in low-grade glioma patients [4–7].
In the literature describing surgical outcomes for glioma patients, the terminology is overlapping. Some authors describe gross total resection as a clinical endpoint achieved as removal of all visually apparent tumor tissue [8]. Others use gross total resection to refer to the lack of enhancing or abnormal residual tumor based on postoperative MR imaging [6]. The term radiographically complete resection is also used to describe an outcome in which there is no evidence of residual tumor on postoperative imaging [9]. Defining residual tumor itself is complicated as well. Some investigators define residual as T2 abnormality, while others define residual tumor as contrast-enhancing tissue. On a microscopic level, glioma cells are known to exist outside of radiographic abnormalities [10, 11]. Consequently, clinical and radiographically defined endpoints for glioma surgery universally underestimate the true extent of disease.
Nonetheless, currently, the highest quality data supporting aggressive resection of low-grade gliomas resection are based on retrospective volumetric analyses of surgical outcomes. These studies suggest that in low-grade gliomas, extent of resection is correlated with progression-free survival, symptom-free survival, and overall survival even after adjusting for patient age, Karnofsky performance score, tumor location, and histologic subtype [12–14]. In the largest published series of hemispheric low-grade gliomas (n = 216), in which extent of resection has been analyzed volumetrically, 8-year survival for patients with radiographically complete resection was 98 %. In contrast, for patients with less than 90 % extent of resection, 8-year overall survival was 60 %. In addition, 8-year progression-free survival rates were doubled for patients undergoing radiographically complete resection (48 %) compared with those whose extent of resection was less than 90 % (21 %) [12]. Mortality among low-grade glioma patients results predominantly from malignant degeneration of residual tumor [15]. Several studies have correlated greater extent of resection with longer malignant progression-free survival [12, 13, 16].
Initially, for high-grade gliomas, because patient outcome seemed poor regardless of the treatment and because of the diffuse infiltration of high-grade glioma cells throughout the brain [17], the benefit of subjecting a patient to extensive surgical intervention was questioned. Recent retrospective analyses have confirmed the importance of resection in maximizing survival for patients with high-grade gliomas [18, 19]. Because ethical and logistical factors have precluded the completion of a definitive randomized clinical trial evaluating the utility of resective surgery for gliomas, the debate continues [20].
Nonetheless, in high-grade gliomas, extent of resection has recently been correlated with survival in volumetric analyses [21, 22] and clinical trials [23]. The largest retrospective volumetric analysis of 500 consecutive patients with newly diagnosed glioblastoma multiforme (GBM) demonstrates a stepwise improvement in outcome with increasing extent of resection when at least 78 % of the tumor can be removed [21]. As in low-grade gliomas, extent of resection was shown to be an independent predictor of overall survival in patients with high-grade gliomas. In addition, in this series, the median survival of patients undergoing radiographically complete resection (16 months) was 30 % greater than the median overall survival of the study population (12 months) [21]. Interestingly, a prospective clinical trial evaluating the utility of fluorescence-guided high-grade glioma resection demonstrated a 4.9-month improvement in survival in patients undergoing radiographically complete resection compared to those with subtotal resections [24]. In comparison, the best adjuvant chemotherapeutic regimen for GBM improves survival 2.5 months [25].
Disparity Between Optimal and Commonly Achieved Surgical Outcomes
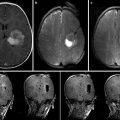
An unfortunate disparity exists between the data supporting maximal resection and the surgical outcomes that are commonly achieved. The clearest explanation for this disparity is that there is no reliable method for delineating tumor tissue that should be removed. In essence, neurosurgeons cannot remove what they cannot see. Today, the most widely used approach for determining completeness of resection in glioma surgery is based entirely on the judgment of the surgeon. Cues such as tissue texture and discoloration, bleeding and vascularity of resection planes, and proximity to anatomic landmarks are used to judge completeness of resection [26, 27]. The subjective nature of judging tumor margins based on gross cues yields highly inconsistent surgical outcomes [12]. Magnetic resonance imaging (MRI)-demonstrable tumor is left behind in up to 70 % of the cases in which surgeons feel that gross total resection has been accomplished. It has been estimated that approximately 20 % of patients receive radiographically complete resections of safely resectable contrast-enhancing gliomas [28, 29].
Even in the minority of cases where all MRI-demonstrable tumors are removed, recurrence is common for low-grade gliomas and universal for high-grade gliomas. This occurs in part because glioma cells can infiltrate into surrounding brain tissue [30] in a manner that cannot be addressed surgically [31]. Interestingly, however, the majority of glioma recurrences occur in or close to the resection bed [32–34]. It is therefore hypothesized that, in many cases, local recurrences arise from resectable tumor left behind following an operation. While it is unlikely that any surgical approach could remove all infiltrating glioma cells in a given patient, it is possible that local control of gliomas could be improved through the use of technologic innovations to confirm the absence of residual tumor in an operative cavity at the end of a resection.
Dye-Based Tumor Delineation
Numerous approaches, some dating back as early as the 1940s, have been developed to overcome the difficulty of detecting glioma and delineating tumor tissue from adjacent normal brain during surgery [35]. For example, a dye-based methods for delineation based on exploiting blood-brain barrier breakdown and metabolic differences between cancerous and noncancerous cells have been proposed [36–42]. The most successful dye to date, 5-aminolevulinic acid (5-ALA), is an orally administered agent, taken up by glioma cells, which convert it to a fluorescent by-product that can be detected with a modified operating microscope [43]. 5-ALA-guided resection was shown in a landmark Phase III clinical trial, to improve extent of resection and progression-free survival in patients with malignant gliomas [23]. However, because dye-based approaches depend on uptake of dye across an abnormal blood-brain barrier in tumors, they are not as effective in low-grade gliomas and in low-grade portions of high-grade gliomas, in which the blood-brain barrier remains intact [44, 45]. A report on a mixed population of 14 primary and metastatic brain tumor patients has suggested a small amount of 5-ALA may reach low-grade glioma cells and be converted to a fluorescent metabolite, protoporphyrin IX. A spectrometric approach can be used to detect the quantity of protoporphyrin IX in a region of interest, thus serving as a biomarker for brain tumor margins.
Image-Guided Strategies
Image-based strategies for maximizing extent of resection, which are noninvasive and eliminate the challenges of delivering dyes to tumors, have also been pursued. Frameless stereotaxy, a nearly ubiquitous neurosurgical tool, has become integral to the planning of brain tumor resections and is covered in depth in Chap. 32 and 33 [8]. However, due to brain shift during exposure, registration inaccuracy, and geometric changes in target tissues between navigational data acquisition time and the time of surgery, frameless stereotaxy has been shown, through a randomized controlled trial, to be ineffective at maximizing extent of resection of brain tumors [46]. Integrating diffusion tensor imaging data into navigational systems may improve safety of tumor resection but has inconsistent effects on extent of resection, depending on tumor grade [47, 48]. Intraoperative high-resolution ultrasound also has an important role in operative planning during brain tumor resection but has limited practical utility in delineating tumor from normal brain [49]. Intraoperative MRI has been shown to be useful in maximizing extent of resection during glioma surgery [6, 50–55] but its high cost has limited wide implementation [56, 57]. Accumulating evidence demonstrating measurable improvements in extent of resection conferred by the use of iMRI for brain tumor surgery suggests that the popularity and adoption of intraoperative MRI will continue to grow.
Intraoperative Fluorescence Microscopy
While gross differences between glioma and normal brain are often subtle, histologic differences are frequently dramatic. Unlike MRI, optical techniques provide the spatial resolution to resolve histologic differences between glioma and normal brain. Traditional histopathologic methods using visible light microscopy cannot be employed in an operative setting to delineate tumor due to the need for toxic stains and the standard arrangement for imaging based on the transmission of light through thin tissue sections. Tissue must therefore be removed for light microscopy to assess for the presence of tumor cells. While the practice of intraoperative histopathologic analysis does not pose a great risk to the patient in most types of cancer, even a small diagnostic biopsy within the brain can result in serious, permanent neurologic deficit [58].
A number of efforts have been undertaken to achieve noninvasive intraoperative cellular-level microscopic imaging with the goal of achieving improved local control of gliomas. Intraoperative confocal microscopy has been recently shown to be feasible for use in the setting of brain tumor surgery [59]. After intravenous or oral administration of fluorescent dyes, a specialized confocal microscope can be used to evaluate a tumor resection cavity for residual tumor cells and interstitial tissue, which has taken up fluorescent dye. Preliminary clinical studies using confocal microscopy to guide brain tumor resection confirm that real-time histologic guidance can be of assistance in safely maximizing extent of resection [8]. While the resolution and contrast of intraoperative confocal microscopy seems adequate, the imaging of residual tumor tissue with confocal microscopy is based on the uptake of fluorescent dyes, which have been known, for decades to have uneven distribution within tumors [35, 44]. Therefore, it is likely that tumor delineation by confocal microscopy methods reliant on fluorescent dyes will have uneven efficacy from patient to patient and within a given tumor.
Label-Free Microscopic Tumor Detection
To avoid the pitfalls of extrinsic contrast agents, label-free methods for intraoperative microscopy have also been proposed. Optical coherence tomography (OCT), in which reflected near-infrared light is used to generate an image, has been shown to be helpful in assessing histology of tissue planes exposed during brain tumor resection. OCT demonstrates the utility of using label-free optical methods for microscopic tissue assessment but generates low-resolution images that can be difficult to interpret, especially in the presence of blood or hemostatic agents in an operative field, leading to false-positive diagnosis of tumor-infiltrated tissue [26].
Third harmonic generation (THG), a nonlinear optical phenomenon, can be used to generate low-resolution, label-free histologic images in brain tissue (Insert third harmonic ref). Contrast within a THG image is based on the microarchitecture of the tissue being imaged with relatively large cell bodies appearing dark, while surrounding neuropil appears bright. The application of THG to a surgical setting has been suggested but has not been reported. While THG can yield information about tissue microarchitecture, it cannot reveal data about the biochemical nature of the tissue being imaged.
There is an increasing body of evidence that suggests that the use of genetic and biochemical markers can improve the accuracy of brain tumor diagnosis and classification. Mass spectrometry enables highly accurate differentiation of tumor from normal brain, as well as specific tumor subtype based on biochemical criteria [60]. Unfortunately, like traditional histopathologic approaches, the use of diagnostic mass spectrometry requires the removal and destruction of tissue for analysis. Similarly infrared spectroscopy has been proposed as a method of delineating tumors based on their biochemical composition. But like mass spectroscopy, IR spectroscopy requires that tissue must be removed and processed for analysis, limiting its use intraoperatively. Removed specimens may, however, be used for routine pathologic analysis. Therefore, techniques that require excision of tissue for analysis may be easily integrated into the standard surgical workflow.
Technical Improvements in Intraoperative Imaging Through Coherent Raman Microscopy
Coherent Raman microscopy represents a major technical improvement beyond currently available methods for maximizing tumor resection. Because coherent Raman microscopy relies on intrinsic biochemical tissue properties to generate contrast within an image, there is no need for visible or fluorescent dyes or labels to delineate tumor. Consequently, the challenge of dye delivery to tumor tissue is eliminated. Moreover, unlike current strategies for intraoperative microscopy, coherent Raman microscopy has the potential to yield both structural and biochemical information about the imaged tissues in situ in a nondestructive manner that may be useful in delineating glioma from normal brain [61].
Unlike intraoperative MRI, coherent Raman microscopy could be easily integrated into the physical and logistical constraints of the standard surgical environment and workflow. The relatively low cost ($100,000) of surgical coherent Raman microscopy systems in preclinical development creates the possibility for wider adoption than currently available methods for maximizing tumor resection. The high cost of intraoperative MRI has been justified by the savings achieved by confirming radiographically complete resection has been achieved at the time of surgery, thereby preventing the need for an additional surgery if residual tumor is detected on postoperative MRI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree