pelvis.1 Passengers in a vehicle struck on the right side are prone to right-sided injuries, including right rib fractures, right lung contusions and lacerations, lacerations of the right lobe of the liver and right kidney, and pelvic fractures. Passengers are less prone to thoracic injuries and more prone to abdominal injuries than are drivers.1
Life-threatening, trauma-specific
Hemoperitoneum
Pneumothorax (lung windows)
Pneumoperitoneum (lung windows)
Hemodynamic status
Active arterial contrast extravasation
Liver and right paracolic gutter
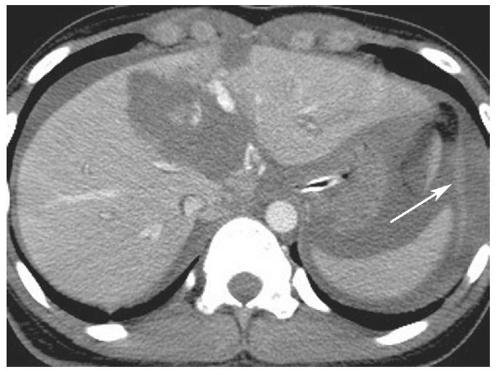
Spleen and left paracolic gutter
Upper abdominal organs, including duodenum and pancreas
Retroperitoneum, including adrenals, kidneys, inferior vena cava, and aorta
Bowel and mesentery
Pelvis
Muscles, including abdominal wall, psoas, iliacus, and buttocks
Bones (bone windows)
Lowest cut (thigh hematoma)
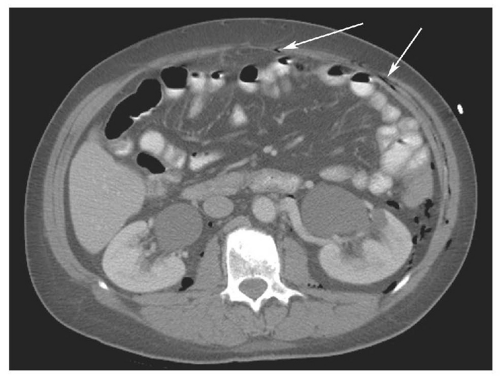
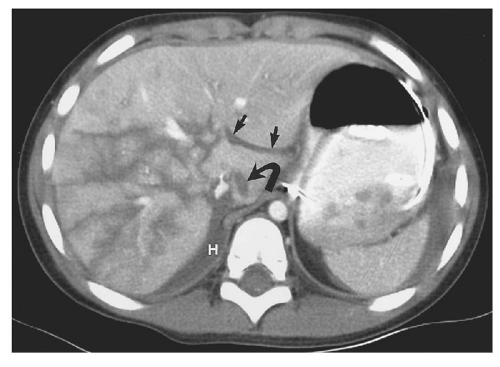
capabilities available with MDCT and is more prone to respiratory motion artifacts. Administration of intravenous contrast medium is mandatory to detect all possible injuries. Unenhanced CT is performed rarely in trauma, limited to patients with a well-documented severe allergy to contrast material. Evaluation of vessels and solid organs is not complete unless intravenous contrast material is administered. Patient preparation is very important but must be accomplished efficiently. To minimize streak artifacts, metal objects within the scanning field should be removed or repositioned, including metal buckles on patient restraints, belts, electrocardiography electrodes (Fig. 15.1) and connecting cables, and metal or metal-reinforced backboards (Fig. 15.2). Some backboards cause such severe artifacts that the patient should be transferred to a more “CT friendly” wooden or plastic backboard. Once the patient is on the CT table, the patient’s upper extremities should be raised above the head to avoid streak artifact. When upper extremity injuries preclude repositioning of the arms, placing the upper extremity close to the patient’s side and enlarging the scanning field to include the upper extremity reduces artifacts. Alert and cooperative patients should hold their breath during the imaging of the upper abdomen to minimize respiratory motion artifacts. However, successful breath holding is often not possible and scanning during shallow, quiet breathing is often necessary.
TABLE 15.1 Grading Criteria for Abdominal and Retroperitoneal Injury | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
1 to 1.25 mm and 3.75 to 5 mm section thicknesses are provided for interpretation. Orthogonal (coronal and sagittal) reformations are generated for all patients for all series acquired, both with 2.5-mm thickness and 2.5-mm reconstruction interval.
Bubbles of free air may be trapped between leaves of mesentery or in the peritoneal recesses. Lung windows are valuable in detecting pneumoperitoneum, which might be overlooked on soft tissue windows because free air may not be distinguishable from adjacent bowel gas at narrow window widths.
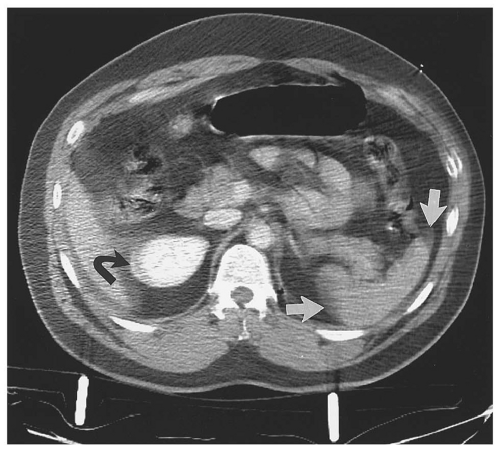
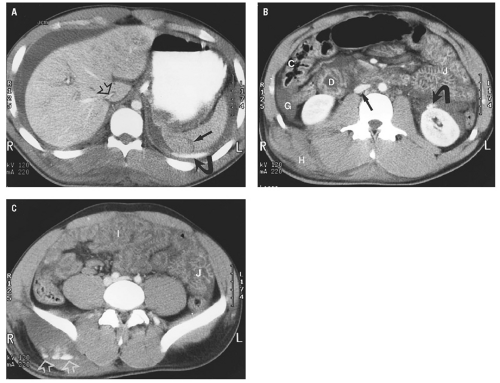
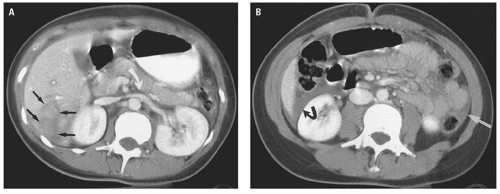
hemoperitoneum may be estimated by searching for fluid in the perisplenic space, perihepatic space, hepatorenal recess, right and left paracolic gutters, and pelvis.46 Small collections of fluid are confined to one space; moderate collections are seen in two or more spaces; and large collections involve all spaces. Although the volume of hemoperitoneum will have an effect on patient management decisions, a large hemoperitoneum does not mandate laparotomy.10, 17
for detecting small amounts of free intraperitoneal fluid has improved, and the radiologist must use all the tools available to help guide further management.
anomalies, or portosystemic shunting. Flattening of the IVC is encountered in outpatients undergoing CT for nontrauma reasons.60 However, in a hemorrhaging trauma patient, a flattened IVC indicates inadequate volume resuscitation. IVC flattening may precede clinical signs of shock. Recognition of intravascular volume depletion on CT should prompt aggressive volume resuscitation before the patient develops more overt signs of cardiovascular collapse.
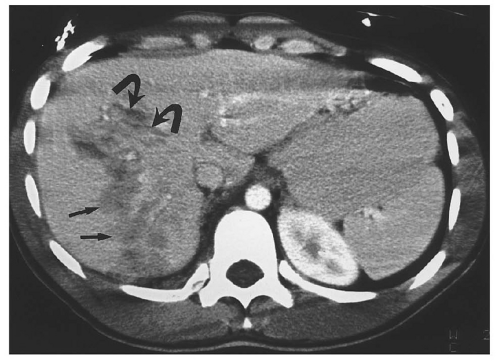
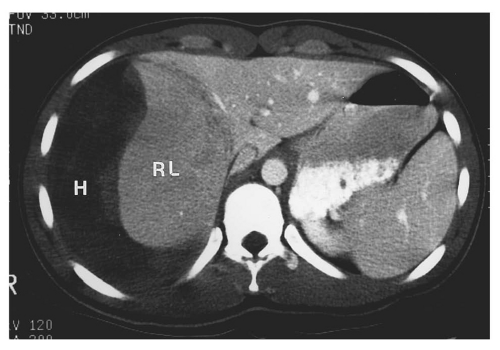
posterior segment of the right lobe most frequently injured.62 Most liver injuries cause hemoperitoneum, but approximately one-fifth do not.63 Liver injuries that do not produce hemoperitoneum will usually be missed by FAST, except in the unusual circumstance that a parenchymal laceration is seen directly by ultrasound. Liver injuries presenting without hemoperitoneum include minor liver injuries that do not cause significant bleeding, injuries that cause purely intraparenchymal hematoma, and injuries that disrupt the surface of the bare area of the liver and cause retroperitoneal hemorrhage.64
to be associated with biliary tract injuries.66 Lacerations extending to the proximal hepatic veins are important to identify because repair of hepatic vein injuries is technically difficult.67
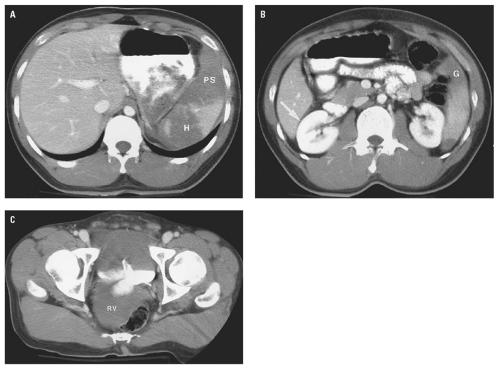
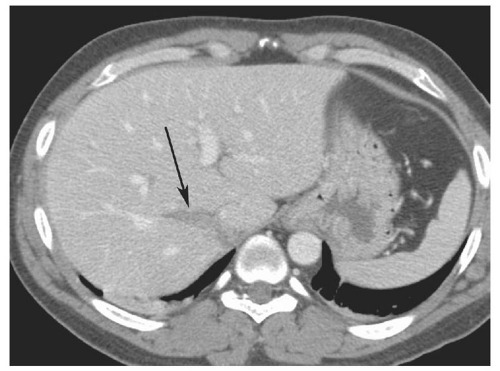
 Figure 15.11. “Bear claw” liver laceration. Complex lacerations involving the left and right hepatic lobes, and extending to the porta hepatis. |
TABLE 15.2 American Association for the Surgery of Trauma Liver Injury Scale (1994 revision) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||