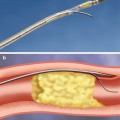
Fig. 36.1
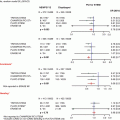
MERCI device. The MERCI device: (a) Left middle cerebral artery thrombus in a patient with acute right-sided weakness (arrow shows thrombus). (b) MERCI device engaged in the thrombus. (c) Angiogram of the left middle cerebral artery post embolectomy with the MERCI device (arrow shows resolution of thrombus)
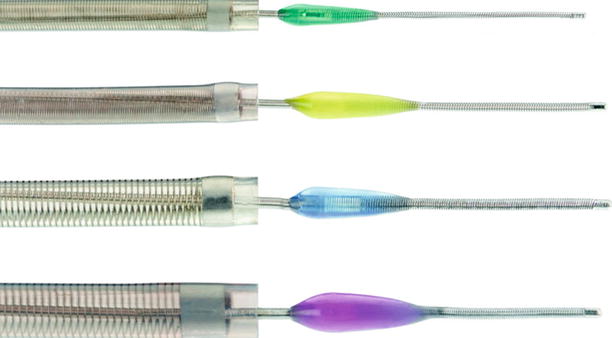
Fig. 36.2
The Penumbra device: Stators with aspiration catheters used for thrombus removal
Mechanical strategies can broadly be divided into several groupings: clot retrievers, aspiration- or suction-type devices, balloons and stents, and ultrasonography-based devices [32]. Ultimately, each of these techniques requires detailed understanding of not only the operational characteristics of the device but also the methods for delivering these potentially bulky tools to the intracranial circulation. As such, injury to the neurovasculature, distal embolization of clot, and conversion of ischemic injury to hemorrhagic injury are all possible complications. Specialized training is required not only to learn the individualized techniques for operation of these devices but also for approaches to selecting the optimal patient population who may benefit from their use.
Intra-arterial Thrombolysis
The concept of intra-arterial (IA) thrombolysis for acute stroke was a logical progression from IV thrombolysis in that it allowed local delivery of the thrombolytic drug directly to the thrombus rather than systemic administration (Fig. 36.3). In theory, this technique offers fewer systemic side effects and more effective thrombolysis. As a result, the therapeutic window for delivery was broader in time scope, as well as patient inclusion [33]. Downsides of this approach include the need for highly skilled interventionalists who can identify the culprit thrombus and successfully navigate the tortuous brain arteries to deliver drug locally as well as appropriate facilities and support staff to allow for successful patient treatment during and after the procedure.
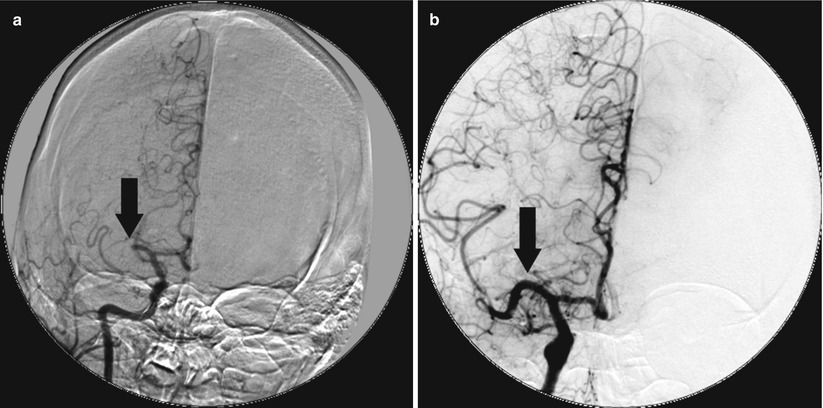

Fig. 36.3
Microcatheter for primary reperfusion. The patient presented with an acute stroke but was lytic ineligible and received catheter-based therapy with local delivery of t-PA via a microcatheter. (a) Occlusion of the right middle cerebral artery (arrow). (b) Reestablished patency of the right middle cerebral artery post-local delivery of t-PA via microcatheter
The PROACT-II trial [25] was a randomized and placebo-controlled trial of patients with middle cerebral artery occlusions and moderately high NIH Stroke Scores (mean 17) who presented within 6 h of symptom onset. The 180 patients enrolled were randomly assigned to treatment with intra-arterial catheter-delivered recombinant prourokinase or low-dose heparin, and standard neurologic outcomes were examined at 90 days, including the modified Rankin Scale score (mRS) which measures functional status. The protocol allowed for placement of a microcatheter up to the thrombotic occlusion, but no mechanical disruption of the clot with wires or catheters. Patients enrolled were highly selected, with over 12,000 patients screened to identify the 180 patients who were enrolled. In this trial, patients who received the thrombolytic therapy had higher rates of artery reperfusion (66 % vs. 18 %) and had better neurologic outcomes as measured by the mRS (40 % had mRS 2 or less in lytic arm versus only 25 % in the control arm). The overall mortality was about 25 % and not different between the two groups, despite a higher rate of intracranial hemorrhage in the patients who received thrombolysis during the first 24 h (35 % vs. 13 %) [25].
Despite the improvements in neurologic outcomes seen in PROACT-II, intra-arterial thrombolytic therapy has not been approved in the United States. Nevertheless, enthusiasm remains for off-label use of commercially available thrombolytics including urokinase and recombinant t-PA, both as stand-alone therapy and when used in combination with intravenous thrombolytics [6]. The concept of a dual thrombolytic approach is appealing in that it allows for rapid treatment of patients in the emergency room setting with IV thrombolytics, followed by local, intra-arterial delivery of additional thrombolytic directly at the site of occlusion (Fig. 36.4). Intra-arterial thrombolysis allows for a higher recanalization rate than systemic intravenous administration, which is less effective in large clots. The Emergency Management of Stroke Bridging Trial (EMS) [34] examined the feasibility and safety of combining intravenous with intra-arterial thrombolytics in patients with acute stroke. In this small study, which enrolled 35 patients with stroke, patients randomized to intravenous and intra-arterial thrombolytics had improved rates of recanalization of infarct arteries compared with those randomized to placebo followed by intra-arterial thrombolysis. Despite no difference in intracerebral bleeding rates, there was no improvement which was noted in overall neurologic outcomes [34]. Since then, other trials have examined the safety endpoints of combined therapy in a nonrandomized fashion, but there has not been overwhelming adoption of this approach [35].
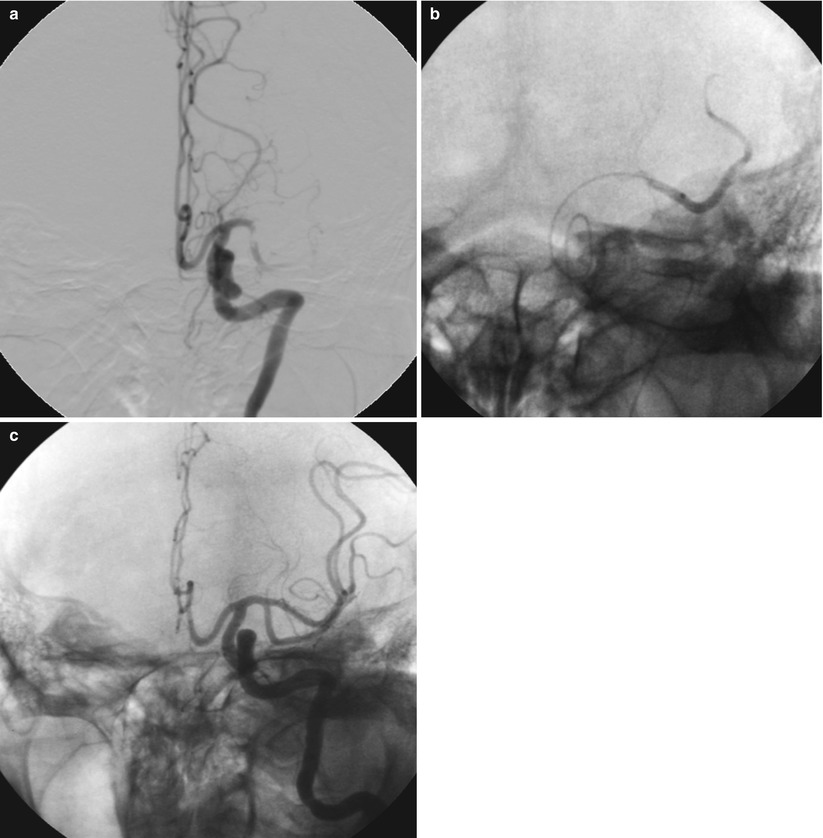

Fig. 36.4
Microcatheter for assisted reperfusion after failed systemic thrombolytics. The patient presented with an acute stroke to an outside hospital and received full-dose systemic thrombolysis. Her symptoms did not improve and she was transferred for rescue intervention. Local delivery of t-PA via a microcatheter allowed for successful reperfusion. (a) Thrombotic occlusion of the left MCA with visible thrombus despite intravenous thrombolytic administration. (b) A microcatheter is delivered distal to the obstruction in the left middle cerebral artery and contrast injection is performed to confirm intraluminal placement. Thrombolytics (t-PA) are then delivered locally to the thrombus via the microcatheter. (c) Final cerebral angiogram demonstrating patency of the left middle cerebral artery after local delivery of thrombolytics. Clinically, this patient had a nearly complete neurologic recovery
The Interventional Management of Stroke II Study (IMS II) [6] used a combined approach of intravenous thrombolysis followed by immediate cerebral angiography with additional intra-arterial delivery of thrombolytics for patients with persistent cerebral artery occlusion. IMS II also employed the EKOS ultrasound catheter for enhanced thrombolysis in a portion of the patients. Overall, this trial found that, when compared with IV thrombolysis alone as in the NINDS trial, the combined approach had no higher rates of intracranial hemorrhage and numerically improved survival when compared to the treatment or placebo arms of the NINDS trial [6]. This encouraging result leads to the organization of the IMS III trial, which is currently enrolling and is randomizing acute stroke patients to intravenous thrombolytic versus combination therapy [36].
Mechanical Thrombectomy
In addition to thrombolytic therapies, there has been an explosion of devices in the past few years which are designed to mechanically remove thrombus from the infarct-related artery. The Mechanical Embolus Removal in Cerebral Ischemia (MERCI) device is FDA approved for the treatment of intracranial clot removal in acute stroke [10]. It has a corkscrew shape and is designed to engage a clot and allow for physical removal of the clot from the cerebral circulation (see Fig. 36.1). It is deployed via a specialized delivery system which is equipped with an occlusion balloon. In theory, this allows for cessation of forward blood flow while the thrombus is being removed and also allows for aspiration of the affected artery so as to prevent further embolization of clot fragments to distal vessels. The pivotal MERCI trial enrolled patients with acute stroke within the first 8 h of symptom onset who were ineligible for intravenous thrombolytic therapy. Successful recanalization was achieved in 48 % of the patients in whom the device was used, with a symptomatic intracranial hemorrhage rate of about 8 % [10]. In patients in whom successful recanalization was achieved, univariate analysis of neurologic outcomes at 90 days showed improvement in mRS, which leads to much excitement about the device and its possible role in thrombolytic-ineligible patients. Interestingly, with multivariate analysis, successful recanalization was no longer a predictor of a good neurologic outcome [37], and there remains much debate about the benefits of the MERCI device and the role that this device should have in the treatment of acute stroke. Newer versions of the MERCI device which incorporate Prolene microfilaments were used in the nonrandomized phase I Multi-MERCI trial, where high rates of successful recanalization were seen when the device was used alone (54 %) or in conjunction with intra-arterial thrombolytics (69 %) [30].
The Penumbra System is another mechanical thrombectomy device which is designed to allow removal of thrombus in the setting of acute stroke. The system consists of an aspiration catheter and a stator which fits through the lumen of the catheter and helps facilitate thrombus maceration and removal (see Fig. 36.2). It was utilized in a single-arm, multicenter registry in 125 patients who presented within 8 h of acute stroke and allowed for successful recanalization in 81 % on the arteries in which it was attempted [38]. Intracranial hemorrhage occurred in 28 % of patients in whom the device was used, with just less than half of hemorrhages classified as symptomatic. Yet despite the high rate of successful recanalization, the rate of favorable clinical outcomes as determined by the mRS was only 25 %, and mortality at 90 days remained high at 33 % [38]. Results such as this have called into question the continued focus on recanalization as a surrogate for success in treating acute stroke, but trials continue to try to define the role for mechanical thrombectomy devices in acute stroke.
Intracranial Stenting
Taking a lesson from the world of coronary intervention, the use of intracranial stents has emerged in the past few years as a means of providing immediate restoration of flow in an acute stroke. Whereas thrombolytics may fail because of the fibrous nature of a more mature embolus, and other mechanical approaches may fail because of embolic adherence to the vessel wall, intracranial stenting is very effective at reestablishing artery patency [39]. Initial attempts at intracranial stenting utilized balloon-expandable stents, which resulted in reasonably high recanalization rates (79 %) but continued high mortality of over 30 % [40]. Part of the difficulty with balloon-expandable stents is the bulky nature of the devices, which have to be delivered to the intracranial circulation via highly tortuous vessels. Moreover, cerebral vessels lack a robust external elastic membrane and have little mobility to stretch and move as a stiff stent travels through because of extensive fixation from branching and perforating arteries [41]. As a result, the chances of vessel damage via dissection or perforation are higher than would be expected when working in the coronary realm. In addition, the consequences of plaque shifting with subsequent compromise of a small perforator artery can be disastrous in the cerebral circulation, making intracranial stenting with balloon-expandable stents a potentially risky proposition.
Because of these unique challenges discussed above, self-expanding stents have gained popularity in the intracranial circulation. They have more flexible delivery systems and are able to track more easily to the target vessel with less risk of vessel injury [42]. There are currently five commercially available stents for use in the intracranial circulation: Wingspan (Stryker, former Boston Scientific, Fremont, CA), Solitaire (ev3, Plymouth, MN), Enterprise (Cordis, Miami Lakes, FL), Leo (Balt, Montmorency, France), and Neuroform (Boston Scientific, Natick, MA). These devices can all be used for the treatment of acute stroke, although they are actually indicated for use in coiling wide-neck aneurysms in the cerebral circulation. Several small single-center experiences with these devices have been reported, including one which included nine patients who were treated just over 5 h after symptom onset and found an 89 % recanalization rate with one intracranial hemorrhage and one acute stent thrombosis [43].
The Stent-Assisted Recanalization in Acute Ischemic Stroke (SARIS) trial is a prospective trial examining the role for stenting in acute stroke [44]. In the trial, 20 patients who either failed intravenous thrombolysis or were not eligible to receive thrombolysis underwent stent-assisted recanalization of the infarct-related artery. Successful recanalization (TIMI 3 or TIMI 2 flow) was achieved in 100 % of the patients, and at 1 month, roughly 60 % of patients had mRS of 3 or less. Many other smaller studies and case series have validated these findings, suggesting that a stent-based approach has real merit in the management of patients with acute stroke [45–47]. An interesting approach which has been utilized in the intracranial circulation is the use of “temporary” stent placement to allow for rapid reestablishment of flow at which point the stent is then retrieved, essentially leaving nothing behind in the intracranial circulation. This approach has several advantages. First, there is no need for dual antiplatelet therapy which can increase the risk of bleeding, particularly intracranial bleeding. Second, stents in the intracranial circulation have been noted to have very high rates of restenosis in small angiographic follow-up studies, although the Wingspan stent used in the SARIS trial had low rates of restenosis at follow-up angiography 6 months after placement [48]. The first case report of temporary endovascular bypass was published in 2008 and detailed a case involving a patient with an unsuccessful mechanical thrombectomy of a large M1 clot where an Enterprise stent was used to successfully reestablish flow to the occluded segment [49]. The stent was left in place for only 20 min and then retrieved from the circulation once flow was restored. The patient did well clinically with a significant fall in NIH Stroke Scale following the procedure. Since then, there have been additional reports which detail similar successes in using stents in a retrievable manner [50]. Based on the high success rates with recanalization and good clinical outcomes, it seems that stent-based therapies for acute stroke hold much promise in the future of stroke therapy.
Neuroprotection
The concept of neuroprotection for acute stroke involves the administration of a drug or other therapy to help minimize ischemic brain injury. While enticing to imagine a drug treatment which blocks the effects of ischemia or protects from cellular damage, the field of neuroprotection has been littered with many “wonder drugs” which held great promise in early animal models yet demonstrated no benefit when studied in man [51]. Many of these drugs are targeted at modulating mediators of neuronal damage, including free radicals, excitatory neurotransmitters, inflammatory markers, and even temperature. There has also been enthusiasm for therapeutic hypothermia in the setting of acute stroke, as it seems to provide benefit following cardiac arrest [52]. However, concerns exist about complications from the required intubation and paralysis which accompanies hypothermia, and the beneficial cerebral effects seen during cooling may be lessened by the requisite rewarming phase [53]. Because of the lack of evidence, there are no current recommendations for administration of neuroprotective agents in the setting of acute stroke, although research continues in this area.
Manpower Issues
Widespread adoption of reperfusion strategies outside intravenous thrombolysis will require a sizable pool of appropriately trained “stroke interventionalists” who are capable of delivering therapy to the brain 24 h a day and 365 days a year. Recent surveys have indicated that fewer than 400 interventional neuroradiologists are currently working in the United States, an inadequate supply to treat even a fraction of the 700,000 strokes which occur in this country every year [54]. Proposals for increasing neuroendovascular manpower have focused on utilizing the pool of carotid-stent-trained interventionalists, a diverse group of providers including neurologists, surgeons, and cardiologists [12]. Specific universal guidelines and training qualifications for acute stroke intervention do not currently exist, but the Society for Coronary Angiography and Interventions (SCAI) is now offering small, multidisciplinary meetings specifically designed to build competence in stroke intervention, including the use of simulators to teach intracranial intervention [55]. More widespread effort to grow the cadre of stroke interventionalists at the community level is the best strategy for impacting stroke outcomes at the national and health policy level.
Despite the multitude of options for the endovascular treatment of stroke, it is clear that not all patients will benefit from this therapy. The role of the “team approach” is to help select the patients who are best suited to catheter-based therapies, determined through assessing the degree of neurologic deficit, duration of symptoms, and size of penumbra as well as incorporating anatomic variables such as degree of collateral circulation. A stroke neurologist is a crucial component of the stroke team and can help guide decision making for the endovascular specialist.
Stroke Centers
Stroke centers are a key component of building a successful stroke program. The concept of stroke centers is relatively new, outlined by the Brain Attack Coalition (BAC) in the year 2000 [56] in response to earlier publications detailing the potential benefits of such centers [57, 58]. The model which has evolved through these efforts is similar to the model for trauma centers or for ST-elevation myocardial infarction (MI). Both trauma care and acute myocardial infarction care share similarities to acute stroke treatment in that they require rapid identification and triage of patients in order to provide timely access to care. This systems-based approach requires community-wide coordination and planning in order to execute in an efficient manner, beginning with empowering ambulances to bypass hospitals in order to bring stroke patients to facilities with appropriate treatment capabilities. To improve stroke care, the BAC has delineated specific components for both Primary Stroke Centers (PSC) [56] and Comprehensive Stroke Centers (CSC) [59]. PSCs are organized to provide a basic infrastructure for acute stroke care, including thrombolytic therapy administration by stroke teams, stroke units, and 24-h access to computed tomography scanning, while CSCs build upon this framework to allow for more comprehensive care.
The components of CSC have been outlined in the BAC consensus document [59




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree