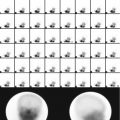
Fig. 9.1
Thirty-two-year-old male with suspected pheochromocytoma. Anterior 131I-MIBG whole-body image with different intensity is shown. There is increased uptake at the supraclavicular region bilaterally. This pattern is due to uptake by brown fat. The remainder of the study shows also physiological distribution of the radiotracer with no abnormalities
Table 9.1
Adrenal hormones and their effects
Structure | Secreted hormone | Functions |
|---|---|---|
Adrenal cortex | ||
Zona glomerulosa | Mineralocorticoids, especially aldosterone | Conserves blood volume through reabsorption of sodium (and water) from distal tubules |
Zona fasciculata | Glucocorticoids, especially cortisol | Increases blood glucose level, stimulates protein catabolism and lipolysis, and increases resistance to stress |
Zona reticularis | Androgens, especially dehydroepiandrosterone | Sustains normal pubic and axillary hair and stimulates RBC production, protein synthesis, and growth |
Adrenal medulla | ||
Epinephrine | More β-receptor effects, e.g., relaxation of musculature of the gastrointestinal tract, uterus, bronchi, and urinary bladder | |
Norepinephrine | More α-receptor effects, e.g., vasoconstriction and contraction of the sphincters and uterus | |
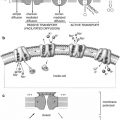
Cholesterol within the adrenal cortex is the starting point for synthesis of multiple adrenal hormones. Therefore, a radioactive cholesterol is useful in evaluating the functional status of adrenocortical lesions. The adrenal medulla is composed histologically of chromaffin cells, which are large ovoid columnar cells arranged in clumps or cords around blood vessels and surrounded by capillaries and sinusoids. They have large nuclei and a well-developed Golgi apparatus; they have a large number of granules containing catecholamines. The adrenal medulla also contains some sympathetic ganglia. The cells of adrenal medulla are innervated by preganglionic sympathetic fibers. Most of the blood supply to the hormonally active cells of the medulla is derived from a portal vascular system arising from the capillaries in the cortex. There is also a network of lymphatics that drain into a plexus around the central vein [2]. The adrenals provide adjustment of heart performance and vascular tone. Epinephrine is found essentially only in the adrenal medulla, constituting greater than 80 % of its output. Norepinephrine is synthesized by adrenergic neurons and cells of the adrenal medulla; therefore, a radioactive norepinephrine analog is used to evaluate adrenomedullary lesions.
9.2 Adrenal Cortex
9.2.1 Pathophysiology
9.2.1.1 Primary Aldosteronism (Conn’s Syndrome)
In primary aldosteronism (Conn’s syndrome), there is increased production of aldosterone by abnormal zona glomerulosa (adenoma or hyperplasia) leading to hypertension through the increased reabsorption of sodium and water from the distal tubules. A benign adenoma accounts for 75 % of cases of this syndrome; it is usually small, ranging from 0.5 to 1.5 cm in diameter. It is more common in women than in men (3:1) and usually occurs between the ages of 30 and 50 years. Bilateral, or rarely unilateral, micro- or macronodular adrenal hyperplasia accounts for most of the remaining cases. Two types of familial hyperaldosteronism have recently been identified: Type I is glucocorticoid suppressible and associated with bilateral hyperplasia, and type II is associated with adrenocortical adenoma. Adrenal carcinoma is a very rare cause of this syndrome. The patients typically come to medical attention because of clinical signs of hypokalemia or detection of previously unsuspected hypertension during the course of a routine physical examination. The diagnosis is principally a biochemical one (low plasma renin activity and a high level of aldosterone); imaging is required to localize the lesion and identify its multiplicity. The diagnostic information provided by CT or MRI in localizing adenomas is both accurate and practical and they are the initial approach of choice. Some smaller adenomas which are not clearly visualized by CT can be depicted by scintigraphy.
9.2.1.2 Cushing’s Syndrome
The most common (60–70 % of cases) pathological cause of this syndrome is the stimulation of the zona fasciculata by excess ACTH from the pituitary gland (Cushing’s disease) or, less commonly, the ectopic production of ACTH (as in small cell lung cancer and neural crest tumors) or corticotropin-releasing factor (CRF) (as in bronchial carcinoid and prostate cancer). Stimulating this zona may lead to bilateral adrenocortical hyperplasia, which is nodular in 25 % and diffuse in 75 % of cases. Cushing’s syndrome may also be due to autonomous adrenal cortisol production (30–40 % of cases) due to adrenal adenoma, or hyperfunctioning adrenal carcinoma. ACTH-induced Cushing’s disease is more common in adults (25–45 years) and is at least three times more common in women than in men. Cushing’s disease resulting from ectopic ACTH secretion is more common in older adults, particularly men. Adrenal tumors rather than pituitary tumors are more common in children, especially girls. Twenty percent of nonfunctional adrenocortical carcinomas tend to be highly malignant, with weights exceeding 1 kg.
9.2.1.3 Hyperandrogenism
Hyperandrogenism can be the result of hypersecretion of androgens (causing virilization) or estrogens (causing feminization) from the zona reticularis of the adrenal cortex by primary adrenocortical hyperplasia and rarely by adrenal tumors, though the most common cause of this syndrome is polycystic ovary disease (POD). In POD, the chronic anovulation associated with increased circulating LH levels results in increased ovarian stromal stimulation, which leads to increased ovarian androgen production. A testosterone-secreting adrenal adenoma may contain the crystalloids characteristic of Leydig’s cells [3].
9.2.2 Scintigraphy
9.2.2.1 Radiolabeled Cholesterol Analogs
NP(131I 7-iodomethyl-19-norcholesterol)-59 (NP-59) is the classic nuclear medicine study used to evaluate some disease processes related to the adrenal cortex. Its main uses are documented cases of adrenal excess secretion and negative or equivocal CT or MRI findings. This radiopharmaceutical is a cholesterol analog that is bound to and transported by low-density lipoproteins (LDL) to specific LDL receptors on adrenocortical cells; therefore, endogenous hypercholesterolemia may limit the number of receptors available for radiocholesterol localization through competitive inhibition. Once liberated from LDL, NP-59 is esterified but is not further converted to steroid hormones [3]. This scan should be done only on patients with clinically hyperfunctioning adrenal cortex verified by lab results, CT, or MRI.
Patient Preparation
1.
Suppression of the normal adrenal cortex is achieved by oral administration of 1 mg dexamethasone q.i.d. beginning 7 days before and for the duration of the study. This is not required in patients with hypercortisolism.
2.
Stop diuretics, spironolactone, and antihypertensive drugs, if feasible for at least 48 h.
3.
Saturated solution of kalium iodide (SSKI) is given orally in a dose of one drop t.i.d. starting 2 days before and continuing for 14 days to suppress the thyroid uptake of free radioiodine. Patients allergic to iodine can take potassium perchlorate (200 mg every night after meals), starting 1 day before injection of NP-59, for 10 days.
4.
A laxative should be given starting 48 h prior to imaging and continuing till final imaging to diminish bowel activity. Enemas may be required. The dose of 131I-NP-59 is 1 mCi, to be strictly injected i.v. through a secured i.v. line over 2 min. NP-59 background clearance and accumulation in the adrenals occurs slowly, but by day 5, accumulation in the normal adrenals is greater than in other organs. Suppressed patients should be imaged on days 3, 4, 5, and 7. If the adrenals are not seen by day 7, dexamethasone should be stopped and the patient imaged on day 10; nonsuppressed patients are imaged on days 5 and 7. Anterior and posterior projections of the adrenals are obtained; in case of hyperandrogenism, the pelvis and genitalia should be included.
The normal distribution is seen in the liver, gallbladder, and colon. In 90 % of cases, the right adrenal gland is more cephalad and deeper than the left adrenal gland. In two-thirds of normal subjects, the activity in the right adrenal appears greater than that in the left in the posterior projection; this is because the right adrenal occupies a more posterior location than the left adrenal. In some instances the gallbladder can be confused with the right adrenal. In the lateral view, the gallbladder is located anteriorly. In difficult cases, cholecystokinin can clear the gallbladder activity. Interfering colonic activity can be reduced by cathartics. Although count rates are low, single-photon emission computed tomography (SPECT) can be performed and may separate adrenals from gut and liver activity.
In primary aldosteronism, early unilateral increased uptake indicates adrenal adenoma, whereas bilateral increased uptake suggests bilateral adrenal hyperplasia. Pituitary ACTH-producing adenoma or ectopic ACTH secretion can result in bilateral adrenal hyperplasia manifested by bilateral symmetric increased uptake, with ectopic causes producing more uptake of NP-59; this pattern may be asymmetric in the macronodular form of hyperplasia. Adrenal adenoma causes unilateral increased uptake, whereas adrenocortical carcinoma gives rise to bilateral nonvisualization. In hyperandrogenism, early bilateral uptake is compatible with hyperplasia, and early (<5 days) unilateral uptake or markedly a symmetric visualization is indicative of adrenal adenoma.
9.2.2.2 PET/CT
Since adrenal adenomas are relatively common (2–9 %) in the general population, incidental detection of adrenal lesions poses a diagnostic challenge, particularly in patients with a previous clinical history of malignancy [4, 5].
CT is used as the first-line diagnostic modality for screening and determining the nature of the adrenal lesions, and MRI is often performed to further characterize indeterminate masses seen on CT. 18F-FDG-PET can help in differentiating malignant from benign adrenal lesions in patients with proven malignancy or in patients with incidentally detected adrenal tumors on CT or MRI studies [5, 6]. However, some adenomas show increased FDG tracer uptake similar to cancer and some do not. It has been suggested that the functional state of an adenoma is a factor determining the intensity of uptake, with 18F-FDG uptake being increased in functioning adrenal masses [7].
Specific inhibitors of adrenal steroidogenesis, etomidate and metomidate, have recently been used to develop suitable PET tracer. These molecules seem to be suitable as in vivo tracers for specific visualization of the normal adrenal cortex and positive identification of adrenocortical tumors. To date adrenocortical radiocholesterol scintigraphy has been shown to be the most accurate noninvasive imaging technique in differentiating benign cortical adenomas from space-occupying or destructive adrenal lesions.
9.3 Adrenal Medulla
9.3.1 Pathophysiology
9.3.1.1 Pheochromocytoma
Pheochromocytoma is a rare tumor arising from chromaffin cells of the adrenal medulla. Most pheochromocytomas produce excessive amounts of norepinephrine, attributable to autonomous functioning of the tumor, although large tumors secrete both norepinephrine and epinephrine [5] and in some cases also dopamine. Releasing the catecholamine into the circulation causes hypertension and other signs. Other catecholamine-producing tumors (e.g., chemodectoma and ganglioneuroma) may also cause a syndrome similar to that seen with pheochromocytoma. Furthermore, they also produce some active peptides such as somatostatin, ACTH, and calcitonin. Pheochromocytomas vary in size from less than 1 g to several kilograms; in general, they are small, most weighing under 100 g. They are vascular tumors, tend to be capsulated, and commonly contain cystic or hemorrhagic areas. The cells tend to be large and contain typical catecholamine storage granules. Multinucleated cells, pleomorphic nuclei, mitosis, and extension into capsule and vessels are sometimes seen but do not indicate that the tumor is malignant. The chromogranin existing within secretory granules in the tumor tends to form Zellballen (cell balls); these structures are surrounded by sustentacular cells. Five to 10 % of cases are malignant, and malignancy is determined by the only biological behavior of the tumor. It is estimated that 0.1 % of hypertensive patients have pheochromocytoma. More than 90 % of patients with pheochromocytoma exhibit hypertension, which is sustained in two-thirds of patients. These tumors are observed somewhat more frequently in women than in men and at all ages, including infancy; they are most common in the fifth and sixth decades [5].
Although most patients with functioning tumors have symptoms (sweating, palpitation, headache, dyspnea, and anxiety), most of the time, these vary in intensity, and in about half of the patients, they are paroxysmal. Pheochromocytomas are sporadic, but about 10–20 % of cases are familial and arise alone or as part of several hereditary syndromes including multiple endocrine neoplasia (MEN) type IIa and type IIb, neuroectodermal disorders (tuberous sclerosis, von Hippel-Lindau disease, and neurofibromatosis type I), Carney’s syndrome (pulmonary chondroma, gastric epithelioid leiomyosarcoma, and paraganglioma), and McCune-Albright syndrome. Pheochromocytoma can be found anywhere in the sympathetic nervous system from the neck to the sacrum; it is subdiaphragmatic in about 98 % of cases. In 85–90 % of these cases, it is found in the adrenal medulla. In sporadic cases of pheochromocytoma, 80 % of the tumors are unilateral, 10 % bilateral, and 10 % extra-adrenal (paraganglioma). By contrast, two-thirds of those occurring in the context of MEN are bilateral. In children, it is extra-adrenal in 30 % of cases. These extra-adrenal locations are as follows: para-aortic sympathetic chain (8 %), organ of Zuckerkandl at the origin of the inferior mesenteric artery (2–5 %), and gonads, scrotum, and urinary bladder (1 %). Fewer than 10 % of these tumors are malignant and metastasize by lymphatic or hematogenous routes; metastases are usually found in the skeleton, liver, lymph nodes, and lungs [6]. The differential diagnosis includes thyrotoxicosis, migraine, sympathomimetic drug use, menopausal hot flushes, and anxiety disorders. Patients with persistent symptoms and hypertension may develop complications such as nephropathy, retinopathy, myocardial infarction, cerebrovascular accidents, and congestive heart failure.
The diagnosis is confirmed by assay of catecholamines and their metabolites, followed by MRI or CT to localize the lesion; predominant production of epinephrine, when present, suggests an adrenal location. Dopamine excretion is a sensitive indicator of tumor aggressiveness, and a rising plasma or urinary dopamine level is regarded as a poor prognostic indicator.
MRI is somewhat more successful in locating extra-adrenal tumors and has the advantage of providing bright images of pheochromocytoma with T2 weighting in contrast to most other adrenal tumors. Only the smallest tumors or those shielded by clips and other metal objects from previous surgery cannot be detected; in these cases, an MIBG study is indicated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree