Drawing 18.1
Adrenal gland anatomy
The suprarenal glands are responsible for the release of hormones that regulate metabolism, immune system function, and the salt-water balance in the bloodstream; they also aid in the body’s response to stress.
18.1.1 Adrenal Cortex
The adrenal cortex secretes three types of hormones; all adrenocortical hormones are cholesterol-derived steroid compounds:
Mineralocorticoids, which are secreted by the zona glomerulosa, including aldosterone.
Glucocorticoids, which are secreted by the zona fasciculata and less from the zona reticularis, as cortisol.
Adrenal androgen, which is predominantly secreted by the zona reticularis, with small quantities released from the zona fasciculata, as dehydroepiandrosterone (DHEA).
18.1.2 Adrenal Medulla
18.2 Pathogenesis
The adrenal gland trauma (AGT) occurs rarely in traumatic patients: in several studies, the incidence varies from 0.03% to 4.95% of blunt or penetrating abdominal trauma cases [1–5]. This is because of its deep position within the retroperitoneum and also because it is protected by soft tissue that surrounds it [2–4, 7]. Adrenal gland lesions are more likely in patient with high ISS [4]; in fact, they occur in severe trauma as car, motorbike or pedestrian accident, falling from height, and sport accident. For retroperitoneal organs, the most frequent lesion mechanism is compression against a fixed structure. Male sex is more affected than the female [2, 4, 9, 10]. Non-isolated traumatic lesions of the adrenal gland are more frequent than the isolated lesions (4%), precisely because they usually occur in severe trauma. In 75–90% of cases, hemorrhages are unilateral and the most affected gland is the right one [2, 3, 6–8, 11, 12].
Bilateral adrenal gland traumatic lesions are still rarer than the monolateral ones and can cause a severe adrenal failure: in literature, there are a few case reports and limited case series [8, 13].
Associated traumatic injuries can affect all organs, by frequency: liver (43%), spleen (23%), lung (19%), and kidney (18%). Pneumothoraces and hemothoraces can be present (80%), as well as, skeletal injuries (46%) to the rib, clavicle, scapulae, pelvis, hip, and spine [2, 4, 6–8, 14] (Figs. 18.1 and 18.2).



Fig. 18.1
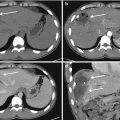
Contrast-enhanced CT. (a, b, and c) Axial scans; (d) sagittal reconstruction. The patient has fallen from height. Adrenal hematoma with associated lesions: axial CT scans show hypodense enlarged adrenal gland (white arrow in a and b); note the presence of associated lesions of liver (black arrowhead in d), upper polar of left kidney (white arrow in d), hemoretroperitoneum, left acetabulum (black arrow in c and d), and lung contusion (white arrowhead in d)

Fig. 18.2
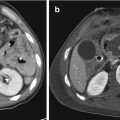
(a and b) Right adrenal hematoma (white arrow), associated with lacerations of the right lobe of the liver (black arrow) and devascularization of the ipsilateral kidney (white arrowhead)
The mortality rates are influenced from the presence of one or more associated lesions.
As has just been said, the right adrenal gland is more involved than the left: this is probably due to anatomical reasons and to their complex vascular supply; in fact, adrenal glands (especially the right one) are susceptible to massive intraglandular bleeding.
Among the causes that make the right adrenal gland more involved in trauma compared to the contralateral, there are three principal theories: the direct compression of the gland between the spine and liver by a direct trauma which affects the upper abdomen, the shearing of small vessels that perforate the adrenal capsule due to the deceleration forces, and also a short-term rise in intraadrenal venous pressure due to compression of the inferior vena cava [3, 5–7, 14–17].
It must be remembered that the right adrenal vein is shorter than the left and communicates directly with the IVC: even for this reason the right adrenal gland is more susceptible to trauma than the contralateral one [2, 4, 5]. In case of right adrenal trauma, a thrombosis in IVC may occur, due to compressive effect from adrenal hematoma or hemorrhage (that causes venous stasis and thus promotes thrombosis).
Adrenal lesions are supposed to result from ischemic necrosis caused by an increase in pressure due to a post-traumatic hemorrhage, or for a direct destruction of the adrenal gland or to both mechanisms [2].
Furthermore, the adrenal arterial supply comes through a sinusoidal net of small arterioles, and the stretch mechanism may easily cut these vessels, resulting in vascular compromise.
Even in pediatric patient, blunt adrenal injuries are uncommon (in 1–4%) and often present as part of a multiorgan trauma in which the most frequently associated lesions are those of liver and ipsilateral kidney. It has multifactorial genesis: accident, sports, child abuse, and so on [14]. It must be remembered that the children have adrenal glands relatively large, for this reason they may be more susceptible to injury due to external compressive forces [18]. Instead in adult, adrenal gland injuries are frequently associated with high injury severity [12] and they are a strong indicator of a possible associated blunt visceral lesion [16, 19].
The injury occurs is more likely in males than in females. The right adrenal gland is most commonly involved [13, 18, 20, 21]. When the right adrenal gland is involved, we have to looking for a liver or right kidney lesions; instead, in case of left adrenal gland injury, we have to consider a possible pancreatic or splenic lesion: in both cases we have to search for a lesion of the lower chest. In case of isolated adrenal injuries, child abuse is a possibility to be considered [17, 22].
18.3 Clinical Findings
There are not specific clinical findings in case of adrenal gland trauma especially in case of monolateral involvement because bilateral lesions that would lead to acute adrenal failure are really rare and because usually adrenal lesion occurs in polytrauma patient and the symptoms are due to multiorgan involvement [7]. There may be nonspecific skin wounds, bruises, or abrasions in the thoracoabdominal region. Anyway, in case of unilateral traumatic adrenal hemorrhages, the symptoms are nonspecific and include abdominal pain, vomiting, nausea, hypo- or hypertension, and decrease in hematocrit [2, 23]. The lack of specific symptoms causes the diagnosis to be often misunderstood, but we have to consider that the presence of adrenal lesion can lead to retroperitoneal persistent hemorrhage and hematoma that can evolve in abscess [24]. In case of bilateral adrenal gland, hemorrhage can cause adrenal insufficiency with “endocrine-related syndromes” which consists in a sudden decrement production of adrenal steroids that cause a state of shock, typically accompanied by a laboratory triad of hypoglycemia, hyperkalemia, and hyponatremia; acidosis-hypotension and lethargy may be present too. If this condition is left untreated, it can be fatal [2, 6, 17, 23]. There are predisposing factors as thromboembolic disease, coagulopathy, pregnancy, and burns [2, 23]. The lack of specific clinical symptoms of an adrenal lesion causes identification to be carried out by diagnostic imaging.
18.4 Imaging
Whole Body Computed Tomography (WBCT) is the choice for the evaluation of the stable polytrauma patient [7, 25, 26] and for traumatic adrenal lesions too. The management of the high energy trauma patient is essentially based on his hemodynamic values and on CT findings [25]. Ultrasonography (US), Contrast-Enhanced Ultrasound (CEUS), and Magnetic Resonance Imaging (MRI) are methods which evaluate the adrenal gland but, in case of trauma, the CT is the method of choice. CEUS and MRI usually can be employed in stable patient, especially during follow-up and in children, according to the radioprotectional criteria [27–29]. Plain radiographs have no value in the detection of traumatic adrenal lesions [4].
18.5 X-Ray
The diagnosis in adrenal trauma fails with conventional radiology for its insufficient diagnostic capacity. The plain X-ray can show bone fractures as associated sign, but there is no correlation between a specific bone fracture and adrenal lesion. In case of previous trauma, X-ray can show the presence of calcifications located in the adrenal space due to a previous hemorrhage. This finding, however, gets into differential diagnosis with neuroblastoma or chronic granulomatous disease.
18.6 Ultrasonography
For its deep position in the abdominal cavity, the adrenal gland is quite difficult to evaluate with US in adult patient: this imaging technique has more chances in children and/or young or thin patient. It is noninvasive, safe and easy to perform, no needs of sedation, it is easily accessible, and relatively inexpensive. US can differentiate cystic from solid adrenal masses and it is useful to assess for vascular involvement but it is often inadequate in the evaluation of the retroperitoneal organs and for eventual complications (as retroperitoneal bleeding) especially in adult patient. Furthermore, it depends on the operator, on the patient’s constitution and his collaboration [23]. In case of severe trauma, US is not the first choice. In case of localized minor trauma, especially in children, CEUS may be used as a valuable method of study [30–32]; furthermore, in case of lesion detection, it must be followed by a complete evaluation performing a contrast-enhanced CT [7]. US and CEUS have high sensibility in detecting even small amount of free fluid in peritoneal space but they are not so able in the evaluation of retroperitoneal free fluid.
US and CEUS can found a role in the follow-up of a lesion previously evaluated with CT or MRI. However, US has specific semeiotics aspects such as the adrenal hematoma and its evolutionary stages over the time [7, 17, 33, 34].



Acute stage: an enlarged hyperechoic adrenal gland, the gland maintains its triangular shape and a cleavage with the upper kidney pole (Fig. 18.3) [4].
Intermediate stage: the mass echogenicity is lower than the previous one, the gland reduces its size, and the mass can assume a complex aspect due to the presence of echoes mixed with anechoic zones (Fig. 18.4).
Chronic stage: the mass is hypo-anechoic with cystic aspect; its size reduces over the time (Fig. 18.5) [4]. The gland can retake its previous morphology and echogenicity or remain with a pseudocystic aspect with a well-defined thin wall [4, 35, 36]; also adrenal atrophy can be seen at the end stage; it is possible to find thin calcifications seen as hyperechoic images in the gland.

Fig. 18.3
Ultrasound exam shows an acute stage adrenal hematoma: the adrenal gland is hyperechoic (calipers), its maintains its triangular shape and the cleavage with the upper pole of the kidney

Fig. 18.4
Ultrasound shows an intermediate stage adrenal hematoma: the gland is enlarged (calipers) with complex aspect, due to the presence of echoic mixed with anechoic zones

Fig. 18.5
Ultrasound shows a chronic stage adrenal hematoma: the gland is still a little enlarged, its echostructure is predominantly hypo-anechoic, with “pseudocystic aspect”
Retroperitoneal or peritoneal fluid may be seen, as well as the other associated lesions of solid organs.
At Doppler examination there is no flow signal in the mass but it is possible to see a peripheral signal due to the capsule. The presence of flow signal within the mass is suspect for a nontraumatic lesion.
The use of contrast medium in US (Contrast Enhancement UltraSonography—CEUS) could easily confirm the absence of contrast enhancement in the adrenal hematoma, delimiting it more accurately than the healthy glandular parenchyma. CEUS may be useful to show the delimitation of the capsule or active bleeding but, about the use of CEUS in adrenal gland traumatic injuries, there are still no scientific data at this time [37–41]. Moreover, CEUS may also be useful in differential diagnosis with other nontraumatic adrenal masses found accidentally and in the follow-up of traumatic and nontraumatic adrenal lesion (Fig. 18.6).


Fig. 18.6
CEUS in patient with blunt abdominal trauma. (a) CEUS shows a hypoechoic adrenal lesion (arrow), which is indistinguishable from a neoplasm. (b) Follow-up, 3 months later: the image is unchanged, demonstrating that it is a little adrenal mass
18.7 Computed Tomography (CT)
CT scan is the best method for evaluating the peritoneum and retroperitoneum especially in emergency conditions, because of its panoramicity, easy accessibility, and rapid scan time [2–5, 7, 23, 42]. In the last years there have been controversies regarding US versus CT in blunt abdominal trauma however, since it is known that US sensitivity is inferior to the CT in detecting all the lesions that can be found in the traumatized patient, US maintains a fundamental role in unstable patient for detecting hemoperitoneum while CT remains the gold standard in stable trauma patient [43, 44].
The most frequent traumatic adrenal gland lesion is hematoma: the gland is enlarged but there is not a disruption of the cortex, often there is periadrenal fat edema or limited hemorrhage [3, 9].
Some CT findings are diagnostic for adrenal hematoma, although others are not distinguished from adrenal neoplasms. It must be remembered that the prevalence of adrenal incidentalomas is 4% in the general population, percentage that increases with age; it is not always easy to differentiate a traumatic adrenal lesion, a traumatic lesion in a pathologic gland or a gland enlargement not related to the trauma [17]. Multidetector CT (MDCT) accurately defines size, location, and shape of adrenal lesions. In addition, it is useful for assessing local and vascular injury. For clear lesions as simple cysts, myelolipomas and often hemorrhage, CT allows a definitive diagnosis because the image is classic. For solid lesions, CT at baseline or in delayed phase may help in distinguishing benign from malignant lesions by their attenuation values: benign lesions tend to have low attenuation values because of an increased fat content [42].
The CT technique used for the evaluation of an adrenal trauma is the same that is used in polytrauma patient. The CT exam has to be performed from “head to toe” with a first acquisition in basal condition followed by two phases: the first in arterial phase and then a venous phase after 60–70 s the beginning of intravenous infusion contrast material or non-ionic iodinated contrast material [6] with the use of a power injector usually through the antecubital vein. The “split-bolus” technique may be useful in avoiding the double phase scan, allowing a reduction in radioexposure [45]. No oral contrast material should be given before scanning.
After the acquisition in the axial plane, post-processing imaging with multiplanar coronal and sagittal reconstruction has been performed [5].
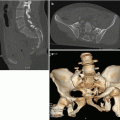
Along with other abdominal organs, also for the adrenal glands the American Association for the Surgery of Trauma (AAST) has drafted a classification that is divided in grades related to the severity of the glandular lesions [9].






Drawing 18.2
Adrenal injury, grade 2

Drawing 18.3
Adrenal injury, grade 3

Drawing 18.4
Adrenal injury, grade 4

Drawing 18.5
Adrenal injury, grade 5

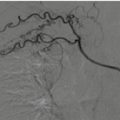
Fig. 18.7
Contrast-enhanced CT, axial scans. (a and b) Arterial phase; (c) venous phase. Widespread hemorrhage of the adrenal loggia, with tearing of the adrenal artery and active bleeding; (c) wide pooling in venous phase
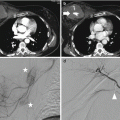
The specific signs of adrenal injury include [2, 3, 5, 6, 9, 13, 17, 46–49]:



- (a)
Round or oval hematoma expanding in the adrenal gland. Oval shape is predominant compared to the round one [4, 7, 14]. At baseline CT, the gland will be enlarged and slightly hyperdense compared to the others abdominal parenchyma. The measured mean attenuation of these lesions are 56 HU ±1 [9, 16, 35]. Instead, in the dynamic phases the gland will be slightly hypodense compared to the others abdominal parenchyma such as liver and spleen [4, 35]: it is possible to see the capsule as a thin hyperdense peripheral rim (Fig. 18.8). The size of the hematoma may vary from 3 cm to more than 10 cm [35]. In case of active bleeding into the hematoma, an hyperdense spot is visible in the enhanced phase (Fig. 18.9).
- (b)
Irregular hemorrhage obliterating the gland.
- (c)
Uniform adrenal gland swelling (Fig. 18.10).
- (d)
- (e)
Adrenal gland rupture (Fig. 18.12).

Fig. 18.8
Motorbike accident. Adrenal hematoma: enhanced CT shows hypodense enlarged adrenal gland, the adrenal capsule is expanse and enhanced (white arrow). Note the associated lesions in hepatic right parenchyma (black arrows), and the presence of perihepatic hemoperitoneum (black arrowhead) and a small amount of free right pleural free fluid (white arrowhead)

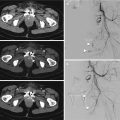
Fig. 18.9
Male, 33 years old, car accident. Unenhanced CT scan (a) shows right adrenal hematoma, as a enlarged gland with slight hypodensity; contrast-enhanced CT, in axial scan (b) and coronal reconstruction (c) show a small slight hyperdensity within the gland (white arrows), which represents a small focus of intraadrenal bleeding

Fig. 18.10




Adrenal hematoma: enhanced CT shows hypodense swelling of the adrenal gland (white arrow); note the thickening of the crus of the diaphragm (white arrowhead)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree