Proton magnetic resonance spectroscopy (H-MRS) may be helpful in suggesting tumor histology and tumor grade and may better define tumor extension and the ideal site for biopsy compared with conventional magnetic resonance (MR) imaging. A multifunctional approach with diffusion-weighted imaging, perfusion-weighted imaging, and permeability maps, along with H-MRS, may enhance the accuracy of the diagnosis and characterization of brain tumors and estimation of therapeutic response. Integration of advanced imaging techniques with conventional MR imaging and the clinical history help to improve the accuracy, sensitivity, and specificity in differentiating tumors and nonneoplastic lesions.
Key points
- •
Proton magnetic resonance spectroscopy (H-MRS) may be helpful in suggesting tumor histology and tumor grade and may better define tumor extension and the ideal site for biopsy compared with conventional magnetic resonance imaging.
- •
Combining H-MRS with other advanced imaging techniques such as diffusion-weighted imaging, perfusion-weighted imaging, and permeability maps improves diagnostic accuracy for intraaxial brain tumors.
- •
Short echo time allows for recognition of more metabolites than long echo time, which is important for differential diagnosis of brain masses and grading tumors.
- •
Higher choline (Cho) levels and lower myoinositol (Myo)/creatine (Cr) ratio are seen in more malignant tumors compared with lower-grade tumors.
- •
Lactate is directly related to tumor grade in adult brain tumors. However, lactate is found in essentially all pediatric brain tumors regardless of histologic grade.
- •
Gliomas are often invasive and show increased Cho levels in surrounding tissues, which may be used to distinguish these lesions from metastases.
- •
When lipids and lactate are found in a solid lesion, lymphoma should be suggested.
- •
A prominent lipid peak is seen in lymphomatosis cerebri, whereas a significant increase in Myo is characteristic of gliomatosis cerebri.
- •
A significant increase in the Cho peak and the presence of lipids and lactate are commonly seen in pilocytic astrocytoma, a grade I tumor.
- •
Typically, higher levels of Cho occur in grade III gliomas; whereas, in glioblastoma multiforme, the Cho levels may be much lower as a result of necrosis.
- •
If the Cho/ N -acetylaspartate ratio is increased outside the area of enhancement, tumor infiltration can be diagnosed.
- •
An increase in Cho-containing compounds after radiation therapy may be seen in radiation necrosis misclassified as tumors.
- •
H-MRS in specific cases improves the accuracy and level of confidence in differentiating neoplastic from nonneoplastic masses.
Introduction
Localized proton magnetic resonance spectroscopy (H-MRS) of the human brain, first reported more than 20 years ago, is a mature methodology used clinically worldwide for evaluation of brain tumors. H-MRS may help with differential diagnosis, histologic grading, degree of infiltration, tumor recurrence, and response to treatment mainly when radiation necrosis develops and is indistinguishable from tumor by conventional magnetic resonance (MR) imaging. Combining H-MRS with other advanced imaging techniques such as diffusion-weighted (DW) imaging, perfusion-weighted (PW) imaging, and permeability maps improves diagnostic accuracy for intraaxial brain tumors.
Introduction
Localized proton magnetic resonance spectroscopy (H-MRS) of the human brain, first reported more than 20 years ago, is a mature methodology used clinically worldwide for evaluation of brain tumors. H-MRS may help with differential diagnosis, histologic grading, degree of infiltration, tumor recurrence, and response to treatment mainly when radiation necrosis develops and is indistinguishable from tumor by conventional magnetic resonance (MR) imaging. Combining H-MRS with other advanced imaging techniques such as diffusion-weighted (DW) imaging, perfusion-weighted (PW) imaging, and permeability maps improves diagnostic accuracy for intraaxial brain tumors.
Technique
Short Echo Time Versus Long Echo Time
Different H-MRS parameters may be optimized and 1 of the most relevant is echo time (TE). Short TE allows for recognition of more metabolites than long TE, which is important for differential diagnosis of brain masses and grading tumors. For example, myoinositol (Myo), a marker for low-grade gliomas, is only seen on short TE acquisitions.
Multivoxel MRS Versus Single-Voxel MRS
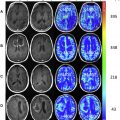
A key consideration for brain tumor evaluations is their metabolic inhomogeneity. Multivoxel (MV) techniques, also called chemical shift imaging (CSI), simultaneously record spectra from multiple regions and therefore map the spatial distribution of metabolites. MV H-MRS provides smaller volumes of interest compared with single-voxel (SV), avoiding sampling error. For these reasons, high-resolution MV MRS such as MRS imaging is often favored for evaluating brain tumors. Nevertheless, SV H-MRS has some advantages compared with MV techniques. SV H-MRS is quicker and easier to obtain in standard clinical settings, providing the opportunity to obtain more than 1 spectrum (ie, spectra at 2 different TEs) in a reasonable amount of time. Evaluating spectra at both short and long TE improves the level of accuracy in differentiating focal brain lesions. SV H-MRS provides better quality spectra compared with MRS imaging. The authors recommend that both techniques be used in the evaluation of brain masses ( Fig. 1 ).
Spectral pattern of tumors
The spectral pattern of intracranial tumors may vary according to histology and malignancy grade and is discussed here.
Reduction in N -Acetylaspartate Levels and in N -Acetylaspartate/Creatine Ratio
Reduction in N -acetylaspartate (NAA) levels and NAA/creatine (Cr) ratio is observed in tumors, indicating decreased viability and number of neurons (see Fig. 1 ; Fig. 2 ). The reader should bear in mind that in low-grade gliomas, the spectral pattern might be similar to that of normal brain ( Fig. 3 ). Absence of NAA in an intraaxial tumor generally implies an origin outside the central nervous system (metastasis) ( Fig. 4 ) or a highly malignant tumor that has destroyed all neurons in that location ( Fig. 5 ).
Decreased Cr Levels
Decrease in Cr may occur, representing energy failure in aggressive malignant neoplasms (see Figs. 1 and 2 ).
Increase in Choline Levels and in Choline/NAA and Choline/Cr Ratios
An increase in choline (Cho) levels is shown by an increase in the Cho/NAA or Cho/Cr ratio, rather than its absolute concentration. Increased Cho is associated with higher turnover in the cell membrane and higher cell density resulting from proliferation of tumor cells (see Figs. 1 and 2 ). In tumors, Cho levels correlate with the degree of malignancy and are linearly correlated with cell density (the inverse of what is seen with the apparent diffusion coefficient [ADC]) instead of the proliferative index. Higher Cho levels are seen in more malignant tumors (see Figs. 1 and 2 ) and lower levels in lower-grade tumors (see Fig. 3 ). Cho is usually higher in the center of a solid mass and decreases peripherally. Cho is consistently low in necrotic areas (see Fig. 5 ).
Myo
Myo is a glial marker because it is primarily synthesized in glial cells, almost only in astrocytes. The Myo/Cr ratio is usually higher in lower-grade (see Fig. 3 ) than in higher-grade tumors (see Fig. 2 ).
Lactate Peak
Increased lactate levels are likely the result of anaerobic glycolysis, although they can also be due to insufficient blood flow leading to ischemia or necrosis. Lactate is directly related to tumor grade in adult brain tumors, with higher peaks seen in higher-grade tumors (see Fig. 2 ). However, lactate is found in essentially all pediatric brain tumors regardless of histologic grade.
Lipids
Increased levels of lipids are believed to be caused by necrosis and membrane breakdown and are observed in metastasis (see Fig. 4 ), aggressive high-grade primary brain tumors such as glioblastoma multiforme (GBM) (see Figs. 1 , 2 , and 5 ) and lymphoma ( Fig. 6 ), and in nonneoplastic lesions such as inflammatory processes and abscesses. A prominent lipid peak is also characteristic for radiation necrosis.
Alanine
Alanine is an amino acid that has a doublet centered at 1.48 ppm. This peak is located above the baseline in spectra obtained with short or long TE and inverts below the baseline on acquisition using TE of 135 to 144 milliseconds. In tumors, an increased level of alanine is considered specific for meningioma ( Fig. 7 ).
Glutamine and Glutamate
Glutamine and glutamate (Glx) and Myo are metabolites better assessed with a TE of 30 milliseconds. Except for meningiomas, in which an increased Glx peak may be seen (see Fig. 7 ), a significant increase in Glx levels should suggest nonneoplastic lesions.
Main clinical applications
Suggest Histology
Although conventional MR imaging is a sensitive modality available for detection of brain tumors, its specificity is low, and several tumor types may share a similar MR imaging appearance. On the other hand, some tumors may present with a typical spectral pattern that may help to suggest the histology.
GBM
The spectral pattern of GBM is typical. There is a significant increase in Cho along with reduction of NAA, Cr, and Myo peaks. Increase of lipids and lactate is also common (see Figs. 1 and 2 ). When there is extensive necrosis, no increase in the Cho peak is seen. In this situation, prominent lipid and lactate peaks may be the only spectral abnormality (see Fig. 5 ). An overlap may be seen between the spectral pattern of GBM and metastasis. Although the absence of NAA in an intraaxial tumor generally implies an origin outside the central nervous system (metastasis) (see Fig. 4 ), a highly malignant tumor that has destroyed all neurons in that location may also demonstrate absence of NAA (see Fig. 5 ). On the other hand, NAA may be present in the spectra of a metastatic lesion if there is a partial volume effect with the adjacent parenchyma. For discriminating solitary metastases from primary high-grade tumors, it has been suggested that investigation of peri-enhancing tumor regions is useful. Metastases are encapsulated and do not show high Cho levels outside the region of enhancement, whereas gliomas are often invasive and show increased Cho in surrounding tissues. However, if tumor infiltration is not significant, no increase in Cho is seen in the peritumoral area surrounding a GBM ( Fig. 8 ).
Meningioma
Meningiomas are readily diagnosed based on conventional imaging features, but the diagnosis may be confirmed by the presence of alanine, which has been reported in many meningiomas. A significant increase in Cho along with some increase in the Glx peak and the presence of alanine are common spectral findings (see Fig. 7 ). Increase in Cho is characteristic and should not suggest malignancy. Meningiomas induced by radiation therapy tend to occur in younger patients, with equal frequency in males and females (sometimes more common in males), and present more atypia and higher nuclear/cytoplasm ratios. In these cases, a large lipid peak along with reduction of all other metabolites including Cho may be seen. Their spectral pattern is similar to that of dural-based metastasis.
Lymphoma
Lymphomas may present as a solitary or multifocal solid lesion with no macroscopic evidence of necrosis in immunocompetent patients. On DW imaging, lymphoma shows hyperintensity with low ADC reflecting a higher nuclear/cytoplasm ratio. The relative cerebral blood volume (rCBV) of lymphomas may be normal to slightly increased compared with the rCBV of high-grade gliomas. The spectral pattern of lymphomas is similar to that of other malignant tumors and is characterized by increase in Cho, reduction in Myo, and prominent lipids. When lipids and lactate are found in a solid lesion, lymphoma should be suggested. The spectral pattern described for solitary and multifocal ( Fig. 9 ) lymphomas is similar to that seen in lymphomatosis cerebri ( Fig. 10 ).
Gliomatosis cerebri
Gliomatosis cerebri is a distinct entity of glial tumors characterized by diffuse infiltration of the glial cell neoplasm throughout the brain. The WHO classification denotes grades II, III and IV gliomatosis cerebri. Therefore, patients with this tumor have a variable prognosis. The most common finding in spectroscopy is reduction of NAA. Increase in Myo is characteristic of gliomatosis grade II, especially if there is no increase in Cho ( Fig. 11 ). Marked increases in Myo and Cr have been found in gliomatosis cerebri and may be attributed to glial activation rather than to glial proliferation because the Cho level is only moderately increased suggesting low glial cell density. Sometimes Cho is reduced (see Fig. 11 ). A prominent lipid peak is seen in lymphomatosis cerebri (see Fig. 10 ), whereas a significant increase in Myo is characteristic of gliomatosis cerebri (see Fig. 11 ). In patients diagnosed with gliomatosis grade III, the Myo peak will be reduced and elevation of the Cho peak will be demonstrated ( Fig. 12 ).
Medulloblastoma
Medulloblastomas are more common in the pediatric population, although they may also present in adults aged 30 to 35 years. They are aggressive tumors (WHO grade IV) with a high propensity to disseminate throughout the cerebral fluid space. Their spectral pattern is characterized by a significant increase in Cho along with a reduction in the NAA and Myo peaks. Some lipids and lactate may be seen. Spectra with short TE show increased taurine at 3.3 ppm in patients. Altering the TE can confirm that a peak at 3.3 ppm corresponds to taurine. At a TE of 30 milliseconds, taurine projects above the baseline, whereas at a TE of 144 milliseconds, the taurine peak is below the baseline. It has been speculated that increased taurine is associated with increased cellular proliferation and tumoral aggressiveness.
Ependymoma
Ependymomas are more common in the pediatric population, although they may also present around the age of 30 to 35 years. They typically occur within the fourth ventricle. A most important imaging finding to identify ependymomas is extension of the tumor through the fourth ventricular outflow foramina ( Fig. 13 ). On computed tomography, the tumor reveals mixed density with punctate calcification in 50% of cases, with variable enhancement. These tumors are heterogeneous on MR imaging, reflecting a combination of solid component, cyst, calcification, necrosis, edema, or hemorrhage. When performed, perfusion MR imaging of ependymoma generally shows markedly increased rCBV and, unlike many other glial neoplasms, poor return to baseline, which may be attributable to fenestrated blood vessels and an incomplete blood-brain barrier (see Fig. 13 C, D). MRS shows considerable heterogeneity. In general, ependymomas have low NAA and moderately increased Cho and Cr. Harris and colleagues stated that the presence of high Myo strongly suggests a diagnosis of ependymoma when short TE (30 milliseconds) is used at 1.5 T (see Fig. 13 E–F). Another study also demonstrated that high Myo and glycine are found in ependymomas, more significant at short TE. According to these findings, when a mass is found in the fourth ventricle, high Myo and glycine suggest ependymoma, whereas high Cho supports primitive neuroendocrine tumor. Sometimes, no increase in Cho is seen in ependymomas (see Fig. 13 F).
Suggest Tumor Grade
Differentiation between high-grade and low-grade tumors is important for therapeutic planning and estimating prognosis. H-MRS may indicate the tumor grade with more accuracy than a blind biopsy, because it assesses a larger amount of tissue than what is usually excised at biopsy.
Tumors are commonly heterogeneous, and their spectra may vary depending on the region sampled by MRS. Hence, the region of interest chosen for analysis has a large influence on the results, and, as stated earlier, MRS imaging is generally considered preferable because it allows metabolic heterogeneity to be evaluated. One recent MRS imaging study used MR perfusion imaging (arterial spin labeling) to guide the spectral measurement location; in regions with increased flow, Cho was found to be higher in high-grade gliomas compared with low-grade gliomas. No metabolic differences between high-grade and low-grade gliomas were found in normal or hypoperfused tumor regions.
H-MRS is considered 96% accurate in differentiating low-grade versus high-grade gliomas. H-MRS may be readily integrated into a multimodality MR imaging examination for presurgical evaluation of patients with gliomas.
Useful metabolites for suggesting tumor grade
Cho
Increased Cho correlates with cellular proliferation and density. There is a high correlation between the in vivo concentration of Cho in brain tumors and in vitro tumor proliferation markers. Statistically significant higher Cho/Cr, Cho/NAA, and rCBV values in high-grade gliomas than in low-grade gliomas have been reported, although threshold values of metabolite ratios for grading of gliomas are not well established. Cho/Cr is the most frequently used ratio. Some institutions use a threshold value of 2.0 for Cho/Cr to differentiate low-grade from high-grade gliomas; others use a cutoff value of 2.5. Although increased Cho is related to tumor grade (higher Cho is found in higher-grade tumors than in lower-grade tumors), some studies have found grade IV GBM to have lower levels of Cho (see Fig. 1 C) than grade II or grade III ( Fig. 14 ) gliomas. This may be due to the presence of necrosis in high-grade tumors, because necrosis is associated with a prominent lipid peak along with reduction of all other metabolites (see Fig. 5 ).
Lipids and lactate
The presence of lipids and lactate correlates with necrosis in high-grade gliomas (see Figs. 1 , 2 , and 5 ). Di Constanzo and colleagues evaluated 31 patients with either high-grade or low-grade tumors through multimodality 3-T MR imaging (including long TE MRS imaging). They concluded that high-grade and low-grade tumors and their margins could be differentiated based on the lactate/lipid signal and rCBV. Lipids are also the main spectral finding in metastasis (see Fig. 4 ). When lipids are demonstrated in solid lesions, lymphoma should also be considered (see Fig. 9 ).
Increase in the lipid peak is inversely correlated to survival.
Lipids and lactate, although usually related to high-grade primary brain tumors and metastasis, may also be demonstrated in pilocytic astrocytomas.
Myo
Useful information on tumor grade may be acquired by using a short TE (30–35 milliseconds) to assess Myo. In low-grade tumors, the Myo/Cr ratio is typically higher (see Fig. 3 ) than in high-grade tumors (see Figs. 1 and 2 ). This may be due to a low mitotic index in low-grade gliomas and, thus, lack of phosphatidylinositol metabolism activation, which results in Myo accumulation. Howe and colleagues concluded that high Myo was characteristic of grade II astrocytomas. Increased levels of Myo have been reported to be useful for identifying low-grade astrocytomas in which the Cho/Cr ratio was not altered.
NAA and Cr
The greatest reductions in NAA and Cr levels occur in higher-grade tumors (compare Figs. 1 and 2 with Fig. 3 ).
Glucose
Short TE spectra may allow the evaluation of a peak around 3.67 ppm (probably glucose), which is directly related to survival. Tumors with more metabolic activity show low glucose levels in the spectra.
Typical spectral findings in grade II, III, and IV gliomas
Grade II gliomas
H-MRS in low-grade gliomas may look similar to normal spectra, demonstrating a discrete reduction in the NAA peak, along with a discrete increase in the Cho peak. An increase in Myo can be the only finding in the spectra of a grade II astrocytoma (see Fig. 3 ). No lipids or lactate are usually demonstrated. Low-grade glioma was studied in vivo at 4 T in 11 patients using H-MRS (incorporating the direct measurement of macromolecules in the spectrum) and 23 Na imaging. The results showed that absolute levels of glutamate and NAA were significantly decreased, whereas levels of Myo and 23 Na were significantly increased in low-grade glioma tissue. The observation of decreased NAA levels is consistent with previous studies. The observed decrease in glutamate contradicts a previous study performed at 1.5 T that suggested that increased Glx maybe characteristic of low-grade gliomas. The discrepancy may be due to the removal of the macromolecule baseline signal intensity in the current study before quantification. The observed increase in Myo is consistent with previous studies.
Grade III gliomas
In grade III gliomas, there is a significant increase in the Cho peak, which correlates well with high cell density in these tumors. The NAA, Cr, and Myo peaks are reduced (see Fig. 14 ). Metastases and glioblastomas nearly always show increased lipid peaks; thus, if the lesion does not exhibit mobile lipid signals, anaplastic glioma is more likely. In the authors’ experience, however, some increase in lipids and lactate may be seen in grade III gliomas ( Fig. 15 A, B ).
Grade IV gliomas
The spectral pattern of grade IV gliomas is characterized by severe reduction of the NAA, Cr, and Myo peaks. Cho is increased (see Fig. 1 C), although not as much as in a grade III glioma (see Fig. 14 C), because a lot of necrosis is usually present in grade IV gliomas, which results in a significant increase in the lipid peak (see Fig. 1 C). Typically, higher levels of Cho occur in grade III gliomas, whereas, in GBM, the Cho levels may be much lower as a result of necrosis. When the voxel is placed within the necrotic area of a GBM, no Cho is detected and a prominent lipid-lactate peak is the only spectral abnormality (see Fig. 5 ).
Special things to remember
Some overlap in the spectral pattern may be seen between grade II and grade III gliomas (see Fig. 15 A–D). Evaluation of the spectra along with the information obtained from the other functional studies such as DW imaging, PW imaging, and permeability maps enhance the diagnostic capacity of brain tumors (see Fig. 15 E–J). Some aggressive tumors, such as metastases, GBM, and gliomatosis cerebri may present with no increase in Cho (see Figs. 4 , 5 , and 11 ). The Cho peak and the Cho/Cr and Cho/NAA ratios may be higher in grade III (see Fig. 14 C) than in grade IV gliomas (see Fig. 1 C). Some benign tumors such as meningiomas present with a significant increase in the Cho peak (see Fig. 7 ). Pilocytic astrocytomas usually present with a significant increase in the Cho peak. Some lipids and lactate are also usually seen in these tumors.
Oligodendroglioma
This tumor is divided into 2 groups according to the WHO classification: grades II and III. It originates from oligodendrocytes but often contains a mixed population of cells, particularly astrocytes. On dynamic contrast-enhanced MR perfusion, low-grade oligodendrogliomas may demonstrate high rCBV because they contain a dense network of branching capillaries. Thus, many oligodendrogliomas can be misinterpreted as high-grade tumors because of their high rCBV.
One study showed that rCBV was not significantly different between low-grade and high-grade oligodendrogliomas. In contrast, another study showed that rCBV was significantly different between low-grade and high-grade oligodendrogliomas. The results of H-MRS studies in oligodendrogliomas are more consistent than those of MR perfusion studies. Similar to astrocytomas, H-MRS of oligodendrogliomas demonstrates significantly higher Cho, Cho/Cr ratio, and a higher incidence of lactate and lipids in high-grade tumors than in low-grade tumors. Nevertheless, low-grade oligodendrogliomas may show highly increased Cho (see Fig. 15 C, D), mimicking high-grade tumors (see Fig. 15 A, B), because these low-grade tumors can have high cellular density but absent endothelial proliferation and necrosis. Apart from higher rCBV, the level of Glx is significantly higher in low-grade oligodendrogliomas than in low-grade astrocytomas and may help to distinguish these tumors from each other.
Assess Tumor Extension
In infiltrative lesions, tumor activity can be demonstrated by H-MRS beyond the enhanced area identified on gadolinium-enhanced conventional MR imaging ( Figs. 16 and 17 ). Comparison of the extent (and location) of active tumor as defined by MR imaging and MRS imaging demonstrates the differences between the 2 techniques. The area of metabolic abnormality as defined by MRS imaging may exceed the area of the abnormal T2-weighted signal. H-MRS may better define tumor extension than conventional MR imaging.