AIRWAY MORPHOLOGY
Airways divide by dichotomous branching. There are approximately 23 divisions from the trachea to the alveoli.
Bronchi are conducting airways that contain cartilage in their walls. The anatomy of the lobar and segmental bronchi is described in
Chapter 6.
Bronchioles are airways not containing cartilage. The largest bronchioles measure about 3 mm in diameter and have walls about 0.3 mm thick. A terminal bronchiole is the last purely conducting airway and does not participate in gas exchange. It is approximately 0.7 mm in diameter and gives rise to respiratory bronchioles. A respiratory bronchiole is the largest bronchiole with alveoli arising from its walls, and, thus, is the largest bronchiole that participates in gas exchange. It gives rise to alveolar ducts and alveoli.
BRONCHIECTASIS
Bronchiectasis is defined as localized, irreversible dilatation of the bronchial tree. Usually, this term is used to refer only to cartilage-containing airways, larger than 2 to 3 mm in diameter.
Etiology
Bronchiectasis has a wide variety of causes (
Table 23-1), but in up to 40% of cases, the etiology cannot be determined. Bronchiectasis is commonly associated with acute, chronic, or recurrent infection, particularly infection with bacteria and mycobacteria. Also, bronchiectasis often is present in patients with bronchiolitis obliterans (BO) or the Swyer-James syndrome resulting from viral or mycoplasma infection. Immunodeficiency syndromes including acquired immunodeficiency syndrome (AIDS), abnormalities of mucociliary clearance, structural abnormalities of the bronchial wall, and chronic bronchial obstruction are associated with bronchiectasis, largely because they result in infection.
Noninfectious diseases that result in airway inflammation and mucous plugging also can result in bronchiectasis. These include allergic bronchopulmonary aspergillosis (ABPA), and, to a lesser extent, asthma. Bronchiectasis also is seen in patients with BO resulting from chronic rejection following lung transplantation or chronic graft-versus-host disease (GVHD) in patients with bone marrow transplantation.
Causes of bronchiectasis having a genetic basis include cystic fibrosis (CF); the dyskinetic cilia (Kartagener’s) syndrome (
Fig. 23-1); Young’s syndrome; Williams-Campbell
syndrome (congenital deficiency of the bronchial cartilage); Mounier-Kuhn syndrome (congenital tracheobronchomegaly); alpha-1-antitrypsin deficiency; immunodeficiency syndromes, including Bruton’s hypogammaglobulinemia, IgA, and combined IgA-IgG deficiencies; and the yellow nail lymphedema syndrome (yellow nails, lymphedema, and pleural effusions).
Pathologic Abnormalities
Bronchiectasis usually is associated with bronchial wall thickening, inflammation, destruction of muscular and elastic elements of the bronchial wall, and bronchial and peribronchial fibrosis. Loss of muscular and elastic tissues allows the bronchi to dilate in response to the pull of adjacent tissues (i.e., the normal negative interstitial pressure). Ciliated epithelium is usually replaced by nonciliated or squamous epithelium. These structural abnormalities of the bronchial wall encourage infection, which, in turn, further damages the bronchi.
Inflammation and fibrosis also are associated with obliteration of small airways and a reduction in the number of bronchial branches, particularly when the disease occurs during childhood, when the lungs are growing.
The affected lung is typically reduced in volume and may be airless. Pulmonary arteries supplying the abnormal lung usually are reduced in number and size, and regional lung perfusion is decreased accordingly.
Clinical Diagnosis
In general, a clinical diagnosis of bronchiectasis is possible only in the most severely affected patients, and differentiation from chronic bronchitis may be difficult. Most patients present with purulent sputum production, recurrent pulmonary infections, fever, weight loss, and sometimes dyspnea. Hemoptysis also is common, occurring in up to 50% of cases, and may be the only clinical finding. Hemoptysis usually is associated with bronchial artery enlargement related to chronic inflammation. Sputum culture often reveals bacterial infection, with common organisms being Streptococcus pneumoniae, Pseudomonas, Haemophilus influenzae, and Staphylococcus aureus. Aspergillus species and mycobacteria also may be present.
Pulmonary function tests may show airflow obstruction. However, bronchitis, bronchiolitis, or emphysema often accompanies bronchiectasis and may predominate as the cause of obstructive abnormalities.
Classification
Traditionally, bronchiectasis has been classified into three morphologic types: cylindrical, varicose, and cystic. The severity of bronchial dilatation and anatomic abnormalities, and, to a lesser extent, functional abnormalities, correlate with these three types. Cylindrical bronchiectasis is associated with the least severe abnormalities, and cystic bronchiectasis is associated with the most severe. However, differentiating between the types of bronchectasis is less important in clinical practice than is a determination of its extent and distribution.
Cylindrical Bronchiectasis
Cylindrical bronchiectasis is characterized by mild bronchial dilatation. The dilated bronchi are of relatively uniform caliber and have roughly parallel walls. Often, smaller bronchi are plugged with purulent secretions. The number of bronchial divisions from the carina to the periphery is slightly reduced.
Varicose Bronchiectasis
With increasingly severe abnormalities of the bronchial wall and increasing bronchial dilatation, the bronchi may assume an irregular, beaded or bulbous configuration, referred to as varicose bronchiectasis. Usually, peripheral bronchi are obliterated by fibrous tissue. The number of bronchial divisions is reduced significantly.
Cystic Bronchiectasis
With severe bronchiectasis, airways appear ballooned or “cystic” or “saccular,” often exceeding 2 cm in diameter. The typical branching appearance of bronchi in areas of bronchiectasis may be difficult to see. The cysts often appear isolated and are not associated with other abnormal airways. Cysts may be seen in a subpleural location. The number of bronchial divisions from the carina to the periphery is markedly reduced.
Bronchography
Although traditionally considered the gold standard for diagnosing bronchiectasis, bronchography has been replaced by CT. The same findings that were considered classic indicators of bronchiectasis on bronchography still can be used in interpreting CT. These include the following:
Proximal or distal bronchial dilatation
Abnormal bronchial contours
Lack of normal tapering of peripheral airways
Reduction in the number of bronchial branches (i.e., “pruning”)
Luminal filling defects due to mucus.
Plain Radiograph Diagnosis
Plain radiographs are abnormal in 80% to 90% of patients with bronchiectasis, although findings are often nonspecific, and the diagnosis can be suggested in only about 40% of patients. The more severe the abnormality, the easier it is to see, and the more likely it is that an accurate diagnosis will be made.
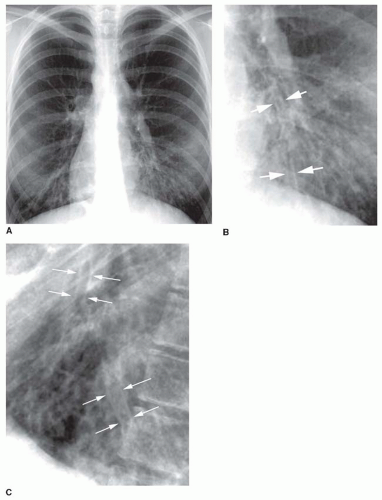
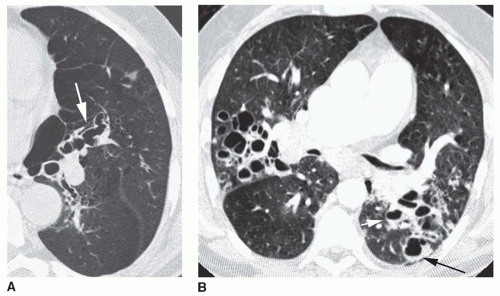
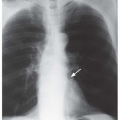
Tram tracks, parallel line shadows representing thickened bronchial walls, are a common finding in bronchiectasis (see
Figs. 23-1 and
23-2). They may be the only finding visible in patients with cylindrical bronchiectasis. However, tram tracks also may be seen with bronchial wall thickening in the absence of bronchiectasis, and thus are nonspecific. When seen in cross section, thick-walled bronchi appear as ring shadows (
Fig. 23-2D).
Recognizing dilatation of bronchi on plain films is more difficult, unless the dilatation is significant or obvious contour abnormalities are present (e.g., in varicose bronchiectasis). The dilated bronchi may be visible as irregular, oval, or branching tubular air-filled structures (see
Fig. 23-2). When seen in cross section, a dilated bronchus may appear larger than the artery adjacent to it. This is known as the
signet-ring sign, and indicates that bronchial dilatation is present. Mucous plugs or fluid filling the bronchi sometimes is visible and may aid in recognizing them as dilated. Mucous plugs within dilated bronchi may be visible as oval (“finger in glove”) or branching (“hand in glove”) opacities.
Cystic bronchiectasis results in multiple, air-filled, cystic lesions, which may be thick- or thin-walled, and clustered, lobar, patchy, or diffuse in distribution. Multiple air-fluid levels often are seen because of infection or retained secretions (see
Fig. 9-28 in
Chapter 9). Because the cysts communicate with each other through the bronchial tree, air-fluid levels tend to be at about the same height in all the affected cysts, but with less fluid present in the upper lobe cysts than in lower lobe cysts (i.e., water flows downhill).
Less specific radiographic findings often seen in bronchiectasis include (1) loss of definition of vascular markings in the affected lung, presumably secondary to peribronchial inflammation or fibrosis, (2) increased “lung markings” in the central lung regions, (3) volume loss in the affected lung or lobe, (4) decreased vessel size in the aerated bronchiectatic lung, (5) consolidation of abnormal lung, and (6) compensatory expansion of normal lung.
CT Diagnosis
HRCT with images spaced at 1 cm intervals or volumetric helical CT using thin collimation (3 cm or less) is very accurate in the diagnosis of bronchiectasis with sensitivity and specificity values exceeding 95%. The use of appropriate and consistent window mean and width settings (-600 to 700/1,000 to 1,500 HU) is important. Excessively low or narrow window settings exaggerate bronchial wall thickness.
Specific findings of bronchiectasis indicate that bronchial dilatation is present (
Table 23-2). Less specific findings include bronchial wall thickening (diagnosed subjectively or because bronchi are visible in the peripheral lung) and the presence of mucoid impaction or fluid within airways. Ancillary findings, commonly seen in bronchiectasis include volume loss, mosaic perfusion visible on inspiratory scans,
focal air trapping identifiable on expiration scans, “tree-in-bud,” and bronchial artery enlargement.
Bronchial Dilatation
Because bronchiectasis is defined by the presence of bronchial dilatation, recognition of increased bronchial diameter is key to the CT diagnosis of this entity. On CT, bronchial dilatation may be diagnosed by an increased bronchoarterial ratio, by detecting a lack of bronchial tapering, and by abnormal bronchial contours.
Increased Bronchoarterial Ratio
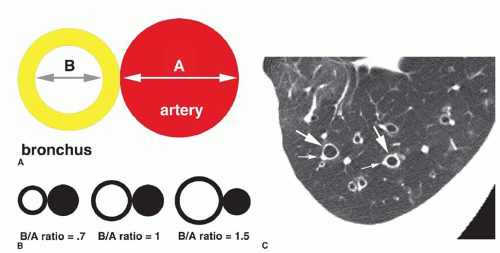
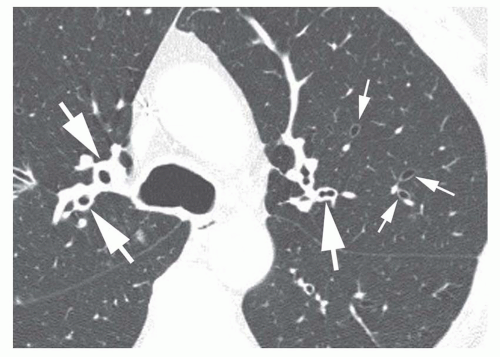
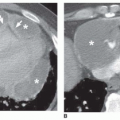
The diameter of a bronchus seen in cross section may be compared to that of the adjacent pulmonary artery by measuring the
bronchoarterial ratio (the ratio of the internal diameter of a bronchus to that of the adjacent pulmonary artery) (
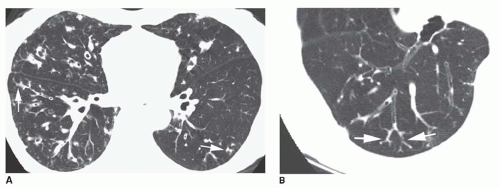
Fig. 23-3A); the bronchoarterial ratio averages about 0.65 to 0.7 in normal subjects.
Bronchiectasis is generally considered to be present if the bronchoarterial ratio exceeds 1. However, this may sometimes be seen in the absence of bronchial wall abnormalities in a small percentage of normals, including elderly patients and in patients living at high altitudes (e.g., Denver). Usually, true bronchiectasis results in a bronchoarterial ratio
greater than 1.5 (
Fig. 23-3B). Unless dilatation is obvious, bronchiectasis should not be diagnosed on the basis of an increased bronchoarterial ratio alone. Fortunately, bronchial wall thickening and other abnormalities typically are present in patients with true bronchiectasis.
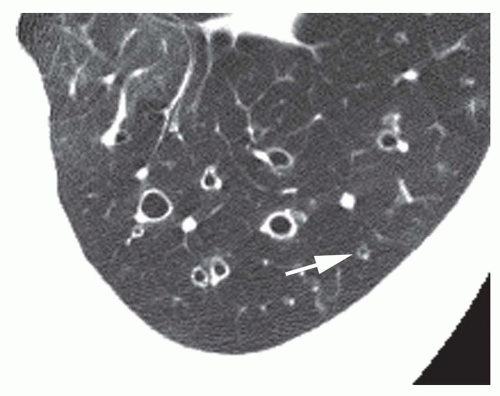
The association of a dilated bronchus with a smaller adjacent pulmonary artery branch has been termed the
signet-ring sign (
Figs. 23-3C and
23-4). This sign is valuable in recognizing bronchiectasis on CT and in distinguishing it from other cystic lung lesions. An increased bronchoarterial ratio not only reflects the presence of bronchial dilatation but also indicates some reduction in pulmonary artery size as a consequence of decreased lung perfusion in the affected lung regions.
Lack of Bronchial Tapering
Lack of bronchial tapering may be important in the diagnosis of bronchiectasis, in particular subtle cylindrical bronchiectasis. For this finding to be present, the diameter of the airway should remain unchanged for at least 2 cm distal to a branching point (see
Fig. 23-4A, C, and D). Accurate detection of this finding is difficult, however, in the absence of contiguous HRCT sections, especially for vertically or obliquely oriented airways.
Abnormal Bronchial Contours
Bronchiectasis may be classified as cylindrical, varicose, or cystic. Recognition of one of these abnormal contours is diagnostic.
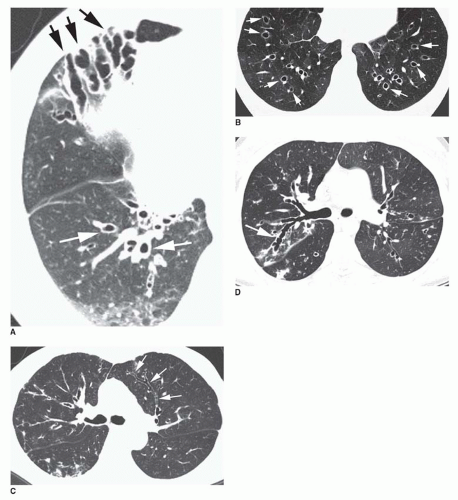
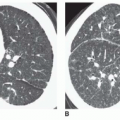
Cylindrical bronchiectasis varies in appearance on CT, depending on whether the abnormal bronchi lie in the scan plane (see
Fig. 23-4A, C, and D) or perpendicular to it (
Fig. 23-4B). When in the plane of scan, bronchi fail to show normal tapering and are recognizable as “tram tracks.”
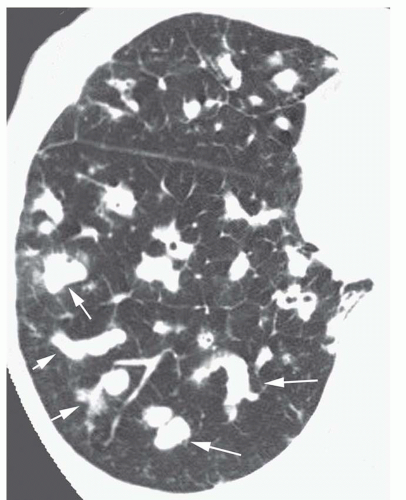
Varicose bronchiectasis results in more irregular bronchial dilatation with a typical varicose appearance (
Fig. 23-5A). The bronchial walls often are irregularly thickened. This diagnosis of varicose bronchiectasis can be made easily only when the involved bronchi course horizontally in the plane of scan. This appearance has been likened to a “string of pearls.” When seen in cross section, the dilated bronchi are visible as thick-walled ring shadows indistinguishable from those of cylindrical bronchiectasis.
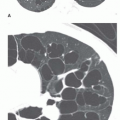
Cystic bronchiectasis is characterized by the presence of numerous cysts (see
Fig. 23-5B). In general, the dilated airways in patients with cystic bronchiectasis are thick-walled; however, the cysts may be thin-walled. A clear-cut branching appearance of the dilated airways generally is lacking, and it may be difficult to make the distinction from cystic lung disease in some cases. This appearance has been described as similar to a “cluster of grapes.” Air-fluid levels may be seen.
Bronchial Wall Thickening
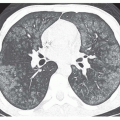
Although bronchial wall thickening is a nonspecific finding, it usually is present in patients with bronchiectasis (see
Figs. 23-3C, and
23-4A and C). Identification of bronchial wall thickening is largely subjective. Because bronchiectasis and bronchial wall thickening often are multifocal rather than diffuse and uniform, a comparison of one lung region to another can be helpful in making this observation (
Fig. 23-6). It must be emphasized that using consistent window settings is very important in the diagnosis of bronchial wall thickening; bronchial walls can vary significantly in apparent thickness with different CT window settings.
Visibility of Peripheral Airways
In normal subjects, airways in the peripheral 2 cm of lung are uncommonly seen because their walls are too thin. Peribronchial fibrosis and bronchial wall thickening in patients with bronchiectasis, in combination with dilatation of the bronchial lumen, allow the visualization of small airways in the lung periphery. Bronchi visible within 1 cm of the costal pleural surfaces indicate bronchiectasis (see
Figs. 23-4A,
23-5B, and
23-7), but bronchi may be seen within 1 cm of the mediastinal pleural surfaces in normal subjects.
Mucous Impaction and Air-Fluid Levels
The presence of mucus- or fluid-filled bronchi is nonspecific, but may be helpful in confirming a diagnosis of bronchiectasis (
Figs. 23-5B and
23-8). The HRCT appearance of fluid- or mucus-filled airways is dependent on both their size and orientation relative to the scan plane. Larger, mucus-filled airways result in abnormal lobular or branching structures when they lie in the same plane as the CT scan. In cross section, impacted bronchi appear similar to vessels, but on contrastenhanced scans they may be seen to be low in attenuation.
Patients with cystic bronchiectasis may show fluid levels within the abnormal bronchi (see
Fig. 23-5B), due to retained secretions and chronic infection. Air-fluid levels within
the multiple cystic spaces are typical, and since they tend to communicate with each other through the bronchi, the air-fluid levels appear similar in height.
Mosaic Perfusion and Air Trapping
Many patients with bronchiectasis also show pathologic findings of small airway disease, either BO or infectious bronchiolitis. Consequently, HRCT findings of small airways disease, such as mosaic perfusion (on inspiratory scans) and focal air trapping (on expiratory scans) are often seen in patients with bronchiectasis.
Tree-in-Bud
The term
tree-in-bud refers to the presence of nodular and Y-shaped branching structures which resemble a budding tree, in the lung periphery (
Fig. 23-9; see also
Fig. 10-28 in
Chapter 10). This appearance reflects the presence of dilated, mucus- or pus-filled centrilobular bronchioles (the trunk and branches) associated with small nodular areas of bronchiolar dilatation or peribronchiolar inflammation (the buds) at the tips of the branches. This finding is common in patients with airway infection, and, therefore, is common in patients with various causes of bronchiectasis.
Bronchial Artery Enlargement
Enlarged bronchial arteries can be identified pathologically in most cases of bronchiectasis. This may result in massive and life-threatening hemoptysis. With multidetector CT using thin collimation and contrast infusion, abnormally enlarged bronchial arteries may be identified; bronchial arteriograms also may be used for diagnosis in patients with hemoptysis. Bronchial artery embolization may be curative in patients with hemoptysis due to bronchiectasis.
Severity of Bronchiectasis
Systems for grading the severity of bronchiectasis using CT have been developed but are not in common clinical use. However, the severity of bronchiectasis may be related to the diameter of the abnormal bronchi. Also, there is a significant correlation between the extent of bronchiectasis
and the forced expiratory volume in 1 second (FEV
1) and the forced vital capacity (FVC). Furthermore, patients with cystic bronchiectasis are more likely to have infection and purulent sputum than are patients with either cylindrical or varicose bronchiectasis.
Pitfalls in the Diagnosis of Bronchiectasis
Several potential pitfalls in the diagnosis of bronchiectasis should be avoided.
Artifacts due to cardiac or respiratory motion may cause ghosting that can very closely mimic the appearance of tram tracks. Observing other artifacts related to motion helps in identifying them appropriately.
Bronchiectasis is difficult to diagnose with certainty in patients with atelectasis. Normal bronchi dilate in the presence of atelectasis because the collapsed lung results in increased tension on the bronchial wall. Bronchi return to normal size when the collapse resolves. This phenomenon is known as “reversible bronchiectasis” (see
Fig. 2-24 in
Chapter 2); this term is an oxymoron, since part of the definition of bronchiectasis is that it is irreversible.
Consolidation may obscure vascular anatomy, making assessment of bronchoarterial ratios difficult or impossible.
Bronchiectasis may occur in association with fibrotic lung diseases (i.e., “traction” bronchiectasis). Traction bronchiectasis does not represent primary airways disease and is not associated with symptoms of chronic infection. The typical corkscrewed appearance of traction bronchiectasis and its association with obvious fibrosis are diagnostic.
SPECIFIC CAUSES OF BRONCHIECTASIS
In most instances, the radiographic appearance of bronchiectasis is nonspecific and does not allow a determination of its cause. However, a few diseases, discussed in the following sections, require special attention.
Cystic Fibrosis
CF is the most common cause of pulmonary insufficiency in the first three decades of life. It results from an autosomal recessive genetic defect in the structure of the CF transmembrane conductance regulator (CFTR), which leads to abnormal chloride transport across epithelial membranes (
Table 23-3). The mechanisms by which this leads to lung disease are not entirely understood, but an abnormally low water content of airway mucus is at least partially responsible, resulting in decreased mucus clearance, mucous plugging of airways, and an increased incidence of bacterial airway infection. Bronchial wall inflammation progressing to secondary bronchiectasis is universal in patients with long-standing disease.
Although a single defect in CFTR is responsible for most cases, more than 100 genetic abnormalities may be associated with CF. These CF genotype variants often cause less severe disease and thus are more likely to be found in adults who first present with symptoms. Individuals who are carriers of the CF gene may have a selective advantage; they appear to be more resistant to typhoid fever than the general population.
Pulmonary abnormalities may be present within weeks of birth, with the earliest abnormalities being retention of mucus in small peripheral airways, mucous gland hyperplasia, and inflammation. The presence of airway infection is fundamental to the development of CF; the first organisms involved are S. aureus, H. influenzae, and mucoid Pseudomonas species. In adults with CF, Pseudomonas aeruginosa predominates (90% of cases), although Aspergillus (up to 50% of cases) species and mycobacteria (up to 20% of cases) also may be present. Bronchial wall damage and bronchiectasis occur largely as a result of infection.
Clinical Presentation
Clinical findings of chronic and recurrent infection in a child, associated with abnormal sweat chlorides, usually are
diagnostic. Hemoptysis occurs in more than half of cases because of bronchial artery hypertrophy.
Radiographic Findings
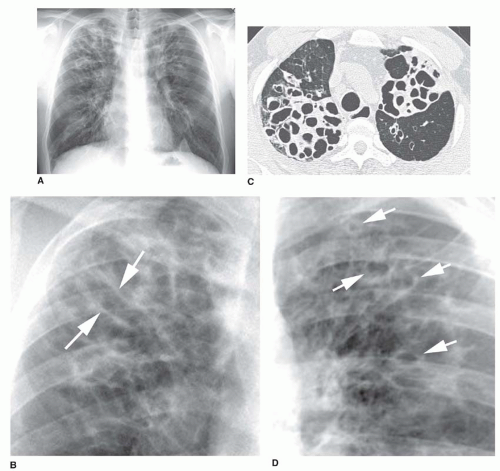
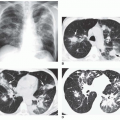
Typical radiographic findings (see
Figs. 23-2 and
23-10) can help confirm the diagnosis in patients with symptoms or may be suggestive in patients with an atypical presentation (e.g., young adults with CF variants).
Early in the course of disease, plain films are normal. Initial abnormalities include the following:
Increased lung volumes due to obstruction of small airways (i.e., increased retrosternal space, flattening of the hemidiaphragms).
Impaction of small airways, resulting in small nodular or reticular opacities in the lung periphery.
Accentuated linear opacities in the central or upper lung regions due to bronchial wall thickening or bronchiectasis.
The earliest abnormalities in patients with CF are in the right upper lobe. Consequently, early findings may include right upper lobe atelectasis or thickening of the wall of the right upper lobe bronchus, best seen on the lateral radiograph.
In older children and adults, bronchiectasis is typical, often involving bronchi in the central lung regions, i.e.,
central bronchiectasis. Dilated bronchi may be cylindrical, varicose, or cystic, depending on the duration and severity of disease. In adult patients and patients with chronic disease, additional abnormalities (see
Figs. 23-2 and
23-10) can include the following:
Mucoid impaction with oval or branching opacities (“finger in glove” or “hand in glove”)
Cysts or ring shadows in the upper lobes, representing cystic bronchiectasis, healed abscess cavities, or bullae
Atelectasis
Pneumothorax (up to 20% of cases)
Hilar enlargement due to lymphadenopathy or pulmonary hypertension
Pleural thickening (although pleural effusion is uncommon).
In most patients with an established diagnosis of CF, clinical findings and chest radiographs are sufficient for clinical management. A significant exacerbation of symptoms may occur with little visible radiographic change.