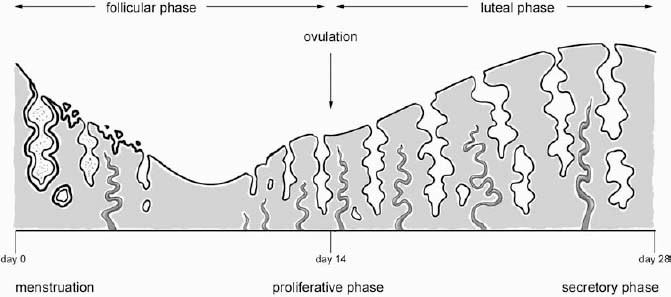
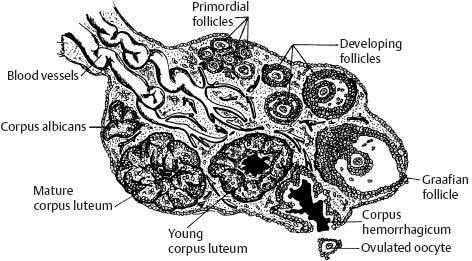
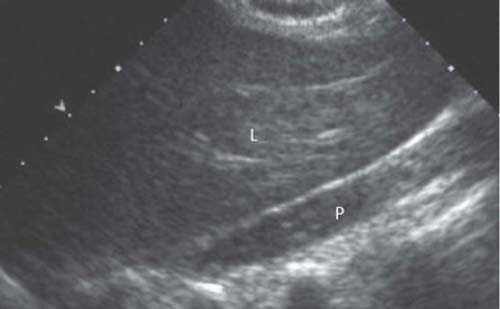
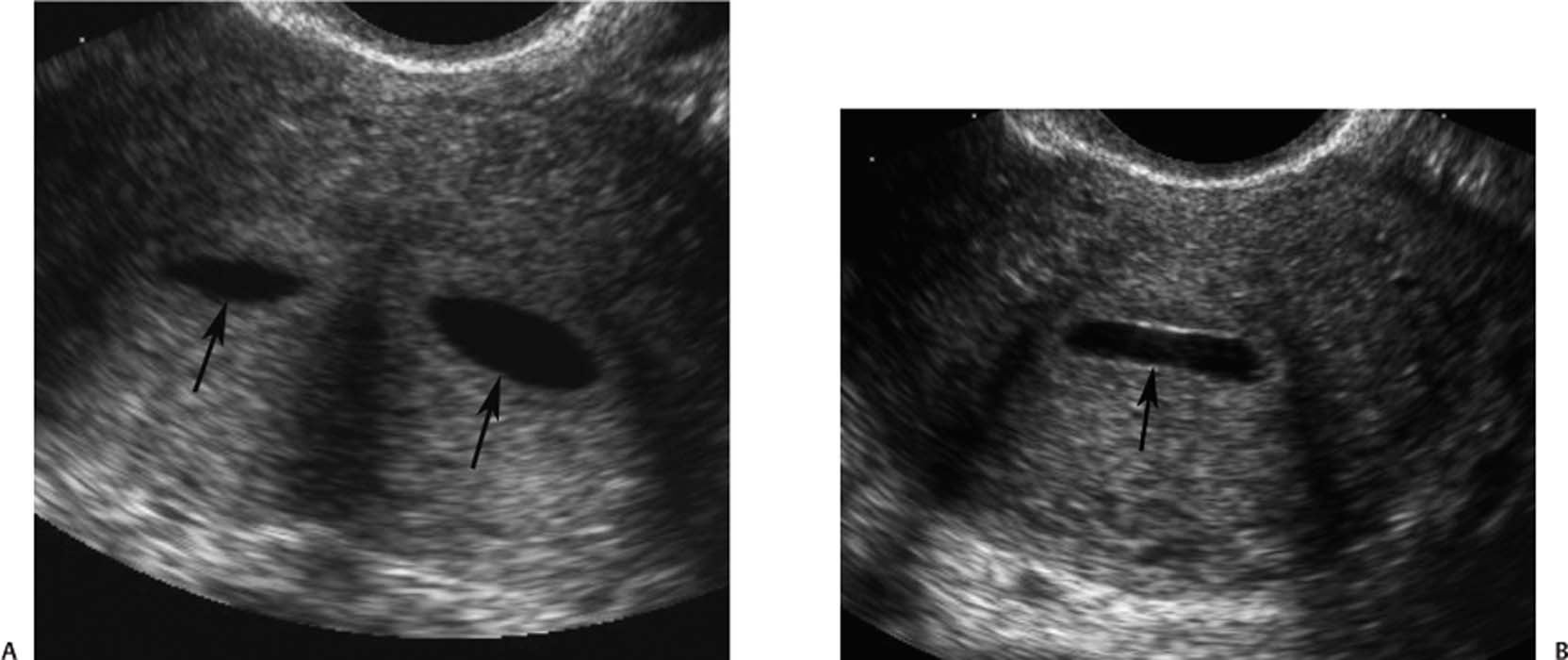
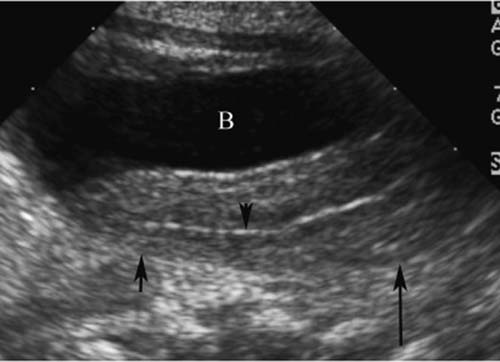
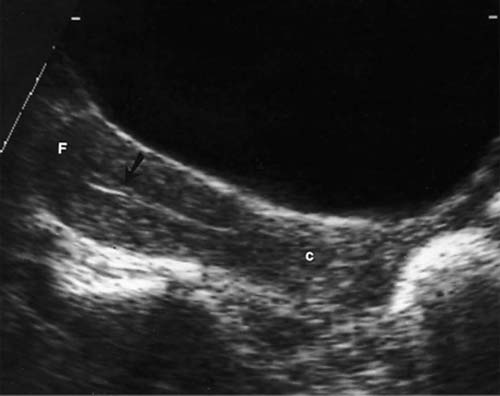
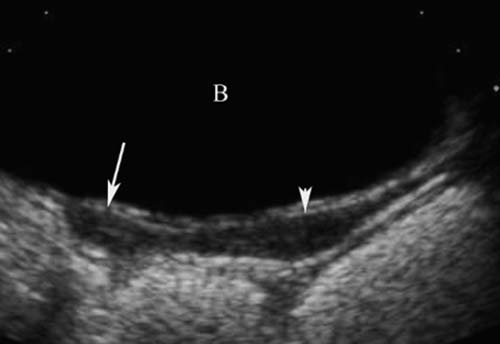
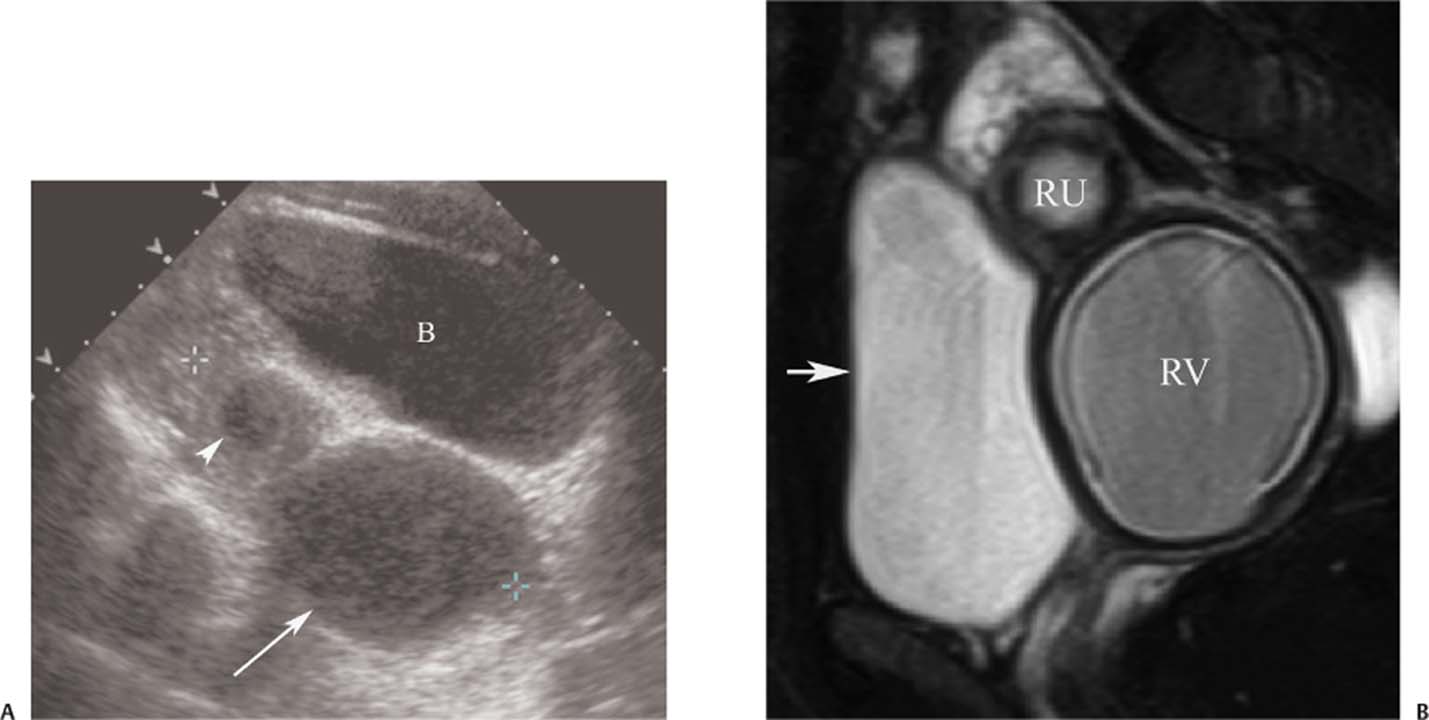
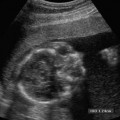
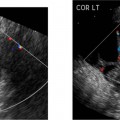
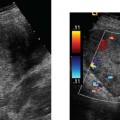
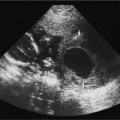
5 Amenorrhea in the Adolescent or Young Adult Ultrasonography of the pelvis and reproductive organs is an essential diagnostic imaging technique for the patient who presents with amenorrhea. Primary amenorrhea is defined as a lack of menses by age 16. Secondary amenorrhea is the cessation of menses at any point in time after menarche and prior to menopause.1 To establish a diagnosis and cause of amenorrhea, the referring physician and radiologist should understand the physiological mechanisms regulating normal menses and the abnormalities that may lead to its disruption. These abnormalities may be embryological, genetic, and/or endocrinologic.2 Knowledge of the patient’s history, physical findings, and laboratory values, as well as the spectrum of normal and abnormal ultrasound and other imaging findings for the patient’s age, is also important for understanding and diagnosing the causes of amenorrhea. Menstruation is the periodic vaginal bleeding that is the end result of cyclical stimulation of ovarian follicles and the associated buildup and eventual shedding of uterine mucosa. In humans, the average time between one menses and the next is 28 days. Stages of the menstrual cycle are defined by the cyclical changes involving the ovary, the endometrium, and hormone levels. With regard to the ovary, the menstrual cycle is divided into follicular and luteal phases, with ovulation occurring 14 days before menses (menstrual bleeding). The first day of the cycle is defined as the first day of menstrual bleeding (menses).1,3–5 With regard to the endometrium, the menstrual cycle is divided into the proliferative and secretory phases, with menses occurring after the secretory phase and before the proliferative phase begins again (Fig. 5–1). The follicular phase of the normal cycle occurs between the first day of the menstrual cycle and ovulation. This phase is variable in length, and accounts for differences in cycle lengths among ovulating women.5 During this phase, low levels of estrogen and progesterone as well as the pulsatile release of gonadotropin releasing hormone (GnRH) from the hypothalamus stimulate the secretion of follicle stimulating hormone (FSH) by the pituitary. This hormone stimulates a group of preselected primordial ovarian follicles (Fig. 5–2). By the sixth day of the cycle, one follicle becomes dominant. With the support of luteinizing hormone (LH) and FSH, this maturing ovarian (graafian) follicle continues to grow and produce estrogen. FSH stimulates an increase in the number of granulosa cells within the graafian follicle as well as the number of FSH receptors on these cells. It also induces production of aromatase, an enzyme necessary for the conversion of androgen precursors to estradiol. LH induces the ovarian theca cells to secrete androstenedione, testosterone, and estradiol into the bloodstream and into the follicle itself. By the midfollicular phase, FSH levels begin to decline and the secondary or nondominant follicles become atretic. Increasing levels of estrogen and the release of inhibin by ovarian granulosa cells contribute to the reduction in FSH production and release. However, the graafian follicle survives because of accumulation of FSH and estradiol in the follicular fluid as well as the earlier increased production of granulosa cells and FSH receptors, which amplify the effect of the available FSH.2,4,5 The last half of the follicular phase coincides with the proliferative phase of the endometrial cycle. Estradiol induces hyperplasia and hypertrophy of the endometrium, with associated thickened mucosa and increased glandular length. Ovulation occurs at midcycle, which is typically at 14 days, but can be anywhere from 9 to 21 days after menses in a normal population. The increasing estrogen and progesterone levels result in a surge of LH. This LH surge causes the distended graafian follicle to rupture and release the ovum (Fig. 5–2). The ovum enters the fallopian tube and either implants in the uterus after fertilization or passes through the uterus and out of the body through the vagina. The length of the next step in the cycle, called the luteal phase, is remarkably constant, averaging 14 ± 2 days in most women and determined by the lifespan of the corpus luteum. During this phase, the ruptured graafian follicle becomes hemorrhagic (corpus hemorrhagicum). The clotted blood is then replaced by lipid-rich luteal cells, which form the corpus luteum. The luteal phase coincides with the secretory phase of the endometrium. After ovulation, estrogen and estradiol produced by the corpus luteum cause the endometrium to become highly vascularized and edematous in preparation for possible implantation by a fertilized egg.5 Figure 5–1 Cyclical changes in the endometrium during a normal 28 day menstrual cycle. The endometrium undergoes changes during a normal cycle. The beginning of menstruation is arbitrarily defined as day 0 of the endometrial cycle. During this phase, there is shedding of the superficial layers of the endometrium noted on the schematic as decreased height of the endometrial lining. Following this, in the proliferative phase, there is hyperplasia and hypertrophy of the endometrium with associated thickened mucosa and increased glandular length. Just after ovulation occurs, anywhere from day 9 to day 21 of the menstrual cycle, the secretory phase begins. It is during this time period that the endometrium becomes highly vascularized with spiral arteries and edematous in preparation for possible implantation by a fertilized egg. The corresponding time periods of the ovary’s follicular and luteal phases are annotated at the top of the drawing, allowing one to note their relationship with the proliferative and secretory phases of the endometrium. (Drawing courtesy of Anna E. Nidecker, M.D.) If pregnancy occurs, the corpus luteum persists and no menses occur until after delivery. If fertilization does not occur, the corpus luteum degenerates (Fig. 5–2), eventually becoming an area of scar tissue (corpus albicans). With corpus luteal degeneration, blood levels of estrogen and progesterone fall. Foci of necrosis appear in the endometrium and soon coalesce. With decreasing hormonal support, the endometrium begins to liberate proteolytic enzymes from lysosomes and increase prostaglandin production. This subsequently causes vasoconstriction and eventual rupture of the spiral arteries of the endometrium, leading to the initially spotty and then more confluent bleeding known as menstrual flow. The stratum functionale, the superficial two thirds of the endometrium fed by the long coiled spiral arteries, is shed. The stratum basale, the deepest third of the endometrium supplied by short straight basilar arteries, remains intact and serves as a regenerative layer for new endometrial proliferation in the next cycle.1,2,4,5 Figure 5–2 Cyclical changes in the postmenarchal ovary. The ovary undergoes various stages during the menstrual cycle. These include the follicular phase with formation of a dominant (graafian) follicle from among several stimulated primordial follicles; the ovulatory phase with formation of the corpus hemorrhagicum; and the secretory phase with formation of the corpus luteum, which produces estrogen and progesterone necessary for endometrial maturation. The corpus luteum degenerates, leaving scar tissue (corpus albicans), if no pregnancy occurs. (From Cohen HL. Evaluation of the Adolescent and Young Adult with Amenorrhea: Role of US. RSNA Special Course in Ultra-sound. Radiological Society of North America; 1996: 171–183. Reprinted with permission from the Radiological Society of North America.) Vaginal bleeding may be noted in the normal female in the immediate neonatal period. It is believed this occasional observation is secondary to withdrawal bleeding from exposure to high levels of maternal estrogen and progesterone in utero, which abruptly decrease after placental separation.2,6 Ordinarily, the final maturation of the reproductive system and subsequent menses do not begin until at least 8 years of age. A central inhibitory mechanism that prevents the pulsatile release of GnRH from the arcuate nucleus of the hypothalamus is thought to prevent menses in younger children. The inhibition of GnRH production in patients with Turner’s syndrome who have no functioning gonadal tissue is evidence for this being due to a central control mechanism, rather than a negative feedback mechanism from ovarian hormone production.7 However, the details of this mechanism remain a mystery. At adolescence, most girls undergo ovarian folliculogenesis without ovulation with the start of the pulsatile release of GnRH.2,8 Unopposed estrogen production leads to progressive uterine growth and endometrial proliferation, as well as physiological leukorrhea and accelerated linear long-bone growth. Thelarche, or breast budding, occurs. Soon afterwards, androgen production by the ovaries and adrenal glands stimulates pubarche, the development of axillary and pubic hair. Finally, menarche occurs, typically 2 to 5 years after breast bud development.2,9 During this time, the hypothalamic–pituitary–ovarian–uterine axis continues to mature. Over an approximately 2-year span, anovulatory cycles with subnormal progesterone production and shortened intermenstrual intervals are gradually replaced by normal ovulatory menstrual cycles.2 The typical ovulatory cycle has a 24- to 35 day interval between menses.10 Longer intervals are often associated with anovulation, although when bleeding occurs, it is often associated with some corpus luteal activity.11 Improved nutrition and living conditions are thought responsible for the gradual decline in mean menarchal ages over the last century. In North America, it is currently 12.4 years, with a range of 9 to 17 years.12 The sex of the embryo is determined genetically at the time of fertilization, but the gonads do not develop sex-specific characteristics until the seventh week of gestation.13,14 The mesodermal urogenital ridges give rise to parts of both the genital and the urinary systems. Because of the association of uterine and renal development, the concurrent presence of abnormalities in both systems is quite common. Thus, if a gynecologic anomaly is found in a patient, one should evaluate for a possible renal or urinary tract anomaly, such as complete renal agenesis (Fig. 5–3), and vice-versa.1,13,15,16 Both sexes develop two different pairs of genital ducts. Components of the wolffian (mesonephric) duct system develop into the epididymis, vas deferens, and seminal vesicles under the influence of testosterone in males. By 6 weeks, a mullerian (paramesonephric) duct has developed lateral to each ipsilateral wolffian duct. In the female fetus, the mullerian duct system (MDS) develops into the fallopian tubes, uterus, and upper two thirds of the vagina, whereas the wolffian system degenerates. External genital development proceeds along female lines in an embryo unless androgens are present.2,13,15 If the MDS is dysgenetic, as in Mayer-Rokitansky-Küster-Hauser syndrome, the uterus or vagina may be absent or rudimentary. Patients with this syndrome have normal karyotypes and normal secondary sex development, but have associated renal (50%) and skeletal (12%) anomalies.2,13,16,17 Figure 5–3 Renal agenesis associated with a congenital gynecologic anomaly. Right upper quadrant ultrasound. Longitudinal plane. The liver (L) and psoas muscle (P), but not the right kidney, are seen in the right upper quadrant of a 17-year-old with a mullerian duct system anomaly and right renal agenesis. If a kidney is not imaged in the renal bed it should be sought by nuclear medicine or other methods to rule out the possibility of an ectopic kidney rather than agenesis. (Image courtesy of SUNY Stony Brook Division of Body Imaging Teaching File.) By 11-weeks gestational age, a Y-shaped uterovaginal primordium has developed into the two fallopian tubes, and fusion of a large portion of the MDS forms a single uterus and upper two thirds of the vagina. This event occurs with or without the presence of ovaries, as long as testes are not present and there are not high levels of androgens in the bloodstream.15 Lack of fusion or variably incomplete fusion of the mullerian duct system can lead to a wide spectrum of anomalies. A complete lack of fusion results in a didelphys uterus, which may consist of two vaginas, two cervices, and two uterine bodies. Partial fusion of only the caudal ends of the MDS results in a bicornuate uterus, characterized by variably separated uterine horns that communicate with a single uterine body, cervix, and vagina. The bicornuate uterus, which is often wider than normal, can be diagnosed on physical exam (usually when the patient is pregnant) by an anterior fundal depression, or sonographically when two endometrial cavities are imaged. The ultrasound diagnosis is most easily made when imaging is performed either during the secretory phase of the menstrual cycle or early in pregnancy, when the endometrial cavity or cavities are most easily seen (Fig. 5–4).1,13,15 Figure 5–4 Bicornuate uterus. Transvaginal sonogram, transverse plane. (A) Two echogenic endometrial cavities (arrows) are noted in this patient whose uterus was imaged during a sonohystogram and whose diagnosis of a bicornuate uterus was an incidental finding. This image does not highlight the sometimes helpful associated finding of an anterior uterine depression where the uterine horns are separated. (B) An arrow points to a single endometrial cavity in the lower uterus. (Images courtesy of SUNY Stony Brook Division of Body Imaging Teaching File.) At the point where the MDS joins the urogenital sinus, the lower third of the vagina develops by elongation of the primitive vaginal plate into a core of tissue that canalizes by week 20.1,15,18 The lumen of the vagina is separated from the urogenital sinus vestibule by a hymenal membrane until late in fetal life. The hymen usually ruptures in the perinatal period, with only a thin fold of mucous membrane remaining around the vaginal entroitus.15,19 Ultrasonography helps in the initial evaluation of patients with amenorrhea by determining whether uterine shape and size are premenarchal (infantile) or postmenarchal (adult). The shape and size of the uterus change during childhood. During the first few months of life, the uterus is influenced by transiently high levels of gonadotropins following placental separation. The typical newborn uterus is shaped like a spade, with the cervical anteroposterior (AP) measurement greater than that of the fundus, and the cervix twice as long as the fundus (Fig. 5–5). In a study of a group of neonates, Nussbaum et al found that the majority (58%) had a spade-shaped uterus. Of the remaining group, 32% had a tube-shaped uterus, with the AP measurement of the cervix equal to that of the fundus. They reported that 10% of their subjects showed the classic adult pear-shaped uterus with a fundus wider than the cervix (Fig. 5–6).19 We have not found the pear-shaped uterus in the normal newborn group. The neonatal uterus has a mean length of 3.5 cm. Transient hormonal stimulation of the neonatal uterus can result in some sonographic findings that are considered more typical of the adult uterus, including an echogenic central endometrial canal with a surrounding hypoechoic halo (seen in 29% of infant girls) and endometrial cavity fluid (seen in 23% of infant girls).1,16 Under the lower gonadotropin levels found typically at ~3 months, the mean uterine length decreases to 2.6 to 3.0 cm.19,20 After the first year of life, the uterus is typically tube-shaped (Fig. 5–7) and remains so through the next several years of childhood.13,16,19 Figure 5–5 Neonatal uterus, longitudinal midline plane. A typical spade-shaped uterus of a newborn is seen posterior to the urinary bladder. The cervix (long arrow) is wider than the fundus (short arrow). A central echogenic endometrial stripe can be seen in the first few weeks of life (arrowhead). Figure 5–6 Normal postmenarchal adolescent uterus. Longitudinal midline plane. In the normal adult uterus, the anteroposterior dimension of the fundus (F) is greater than that of the cervix (c). The central endometrial echogenicity has a triple echogenic line appearance typical of the proliferative phase, rather than the single echogenic stripe typical of the secretory phase. The length of the uterus increases gradually between 3 and 8 years of age, reaching an average measurement of 4.3 cm in the premenarchal child.20–22 The increase in uterine length, its change to a pear shape, and the reversal of the ratio of corpus to cervical length are believed to be a consequence of increasing estradiol levels as well as two other independent variables: patient age and height.20,21 There is a moderate correlation between uterine length and weight (r = 0.69).21 After puberty, the typical uterus measures 5 to 8 cm in length. It descends deeper into the pelvis and may become anteverted or retroverted.1,23 In addition, cyclic endometrial changes can be seen on ultrasound. A well-defined “triple line,” representing the echopenic functional layer and the central echogenic line of the coapted endometrial canal, is indicative of a proliferative endometrium. This triple line is replaced by a single echogenic stripe, representing the meeting of the thickening hyperechoic functional layers during the secretory phase of the endometrial cycle.24 Figure 5–7 Tubular premenarchal uterus, longitudinal midline plane. A normal tubular premenarchal uterus in a 7-year-old girl. The large arrow points to the fundus and the arrowhead points to the cervix. A central endometrial stripe is seen. B, bladder. The identification of the uterus and determination of its size are key pieces of diagnostic information in the evaluation of several pediatric and adolescent gynecologic disorders, including causes of amenorrhea. Ultrasonography can aid in the diagnosis of anomalies of the mullerian duct system, particularly the bicornuate uterus and the uterus with partial or complete obstruction (Fig. 5–8A). Three-dimensional (3-D) ultrasound shows some further promise in the analysis of these anomalies.25 Magnetic resonance imaging (MRI) with its multiplanar imaging has been successfully used to evaluate several of these anomalies (Fig. 5–8B). MRI, because of its accuracy and detail, is the study of choice for the further evaluation of MDS anomalies found on ultrasound, particularly in complex cases.26 For example, MRI can identify the tissue composition of an intrauterine septum, helping to differentiate between a bicornuate uterus with a myometrium-containing septum and a septate uterus with a fibrous septum.27–29 Premenarchal ovaries are best imaged sonographically. Normal premenarchal ovaries are almond shaped and typically found posterior and lateral to the uterus. They are often seen lateral to the uterus on transverse sonographic images while the transducer is angulated in a superior-inferior direction. On parasagittal scanning, ovaries appear medial and anterior to the iliac vessels. Although usually present at the level of the superior portion of the broad ligament, the ovaries may be found anywhere along their embryological course, from the inferior border of the kidneys to the broad ligament.4 The true absence of an ovary is rare. If an ovary and its ipsilateral fallopian tube are found to be absent during surgery, it is more likely due to an antenatal torsion with secondary necrosis rather than ovarian agenesis.1 Concepts with regard to the sonographic analysis of the normal ovary for volume and echogenicity pattern have evolved over the past 15 years through the evaluation of both adults30 and children.31,32 The once classic adult ovary measurement of 3 × 2 × 1 cm (3 cc volume) underestimates the normal mean ovarian volumes now reported in menstruating women, at 6 to 10 cc. At one time, the sonographic demonstration of pediatric ovaries was thought to be difficult and the normal mean ovarian volumes in patients under 10 years of age was reported to be as low as 0.7 cc.32 However, Cohen et al imaged ovaries in 64% of 77 girls from birth to 2 years of age,31 and in 78% of 101 girls between 2 and 12 years.32 Figure 5–8 Vaginal obstruction in mullerian duct anomaly, didelphys uterus. (A) Transverse ultrasound showing the bladder (B) anterior to a fluid-filled obstructed right vagina (arrow) and uterus (arrowhead) of a teenager with two vaginas and two uteruses as part of a müllerian duct anomaly. (B) Sagittal T2-weighted magnetic resonance image to the right of midline shows a fluid-filled bladder (arrowhead) anterior to a fluid-filled obstructed right vagina (RV) in the same patient. RU, right uterus. (Images courtesy of SUNY Stony Brook Division of Body Imaging Teaching File.) Mean volumes for premenarchal girls range between 0.75 cc and 4.18 cc.21,23
Normal Structure, Function, and Development of Female Reproductive Organs
Menstrual Cycle
Physiology
Genital Embryology
The Pediatric Uterus
Imaging Tests to Congenital Uterine Anomalies
The Pediatric Ovary
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree