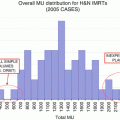
Trial
Initial publication year
# Pts enrolled
Radiation technique
Median treatment duration (days)
Treatment breaks (%)
Median treatment break duration (days)
Acute G3+ heme toxicity (%)
Acute G3+ derm toxicity (%)
Acute G3+ GI toxicity (%)
Acute G3+ GU toxicity (%)
Colostomy free survival or colostomy rate (%)
Overall survival (%)
ACT I (5FU/MMC arm)
1996
292
2D
NA
19
NA
11
17
48
1
CFS-50 (3 YR)
65 (3 YR)
EORTC (5FU/MMC arm)
1997
51
2D
NA
NA
NA
NA
29
10 (diarrhea)
NA
CFS-72 (5 YR)
56 (5 YR) (both arms)
RTOG 87–04 (5FU/MMC arm)
1996
146
2D
NA
NA
NA
G4+ 18
NA
NA
NA
CFS-71 (4 YR)
76 (4 YR)
RTOG 98–11 (5FU/MMC arm)
2008
341
2D
49
62
9
58
45
34
3
CFS-72 (5 YR)
84 (3 YR)
ACCORD 3 (CIS/5FU, no induction arms)
2012
157
3D
NA
NA
NA
13
NA
12 (diarrhea)
NA
CFS-76 (3 YR)
71 (5 YR)
ACT II (5FU/MMC arms)
2013
472
3D
38
14
NA
26
48
16
1
CFS-74 (3 YR)
79 (5 YR)
RTOG 05-29
2013
52
IMRT (DP)
43
49
0
58
23
21
2
CFS-86 (2 YR)
88 (2 YR)
Salama et al.
2007
53
IMRT (DP or SEQ)
42
42
4
59
38
15
0
CFS-84 (1.5 YR)
93 (1.5 YR)
Pepek et al.
2010
19/45 (SCC)
IMRT (SEQ)
NA
18
5
24
0
13
2
CFS-91 (2 YR)
100 (2 YR)
Bazan et al. (IMRT group)
2011
29
IMRT (SEQ)
40
35
1.5
21
21
7
NA
CFS-86 (3 YR)
88 (3 YR)
DeFoe et al
2011
78
IMRT (DP)
NA
66
2
43
29
28
0
CFS-81 (2 YR)
87 (2 YR)
Kachnic et al.
2012
43
IMRT (DP)
NA
40
2
61
10
7
7
CFS-90 (2 YR)
94 (2 YR)
Vieillot et al.
2012
39
IMRT (DP)
NA
15
6
25
42
10
5
CFS-85 (2 YR)
89 (2 YR)
Dasgupta et al.
2013
45
IMRT (DP)
40
NA
NA
NA
NA
NA
NA
CFS-97 (2 YR)
93 (2 YR)
Mitchell et al.
2013
65
IMRT (DP)
NA
9
1.5
3
17
9
2
CR-3 (2 YR)
96 (2 YR)
18.2 Published Data Using IMRT
In 2005, Milano et al. published the first institutional experience of IMRT in the treatment of 17 patients with anal cancer at the University of Chicago. Patients treated with IMRT were compared to those treated with simple AP/PA plans for dosimetric and clinical outcomes comparison. IMRT reduced mean and threshold doses to the small bowel, bladder, and genitals. In the 17 patients treated with IMRT, no grade 3+ acute non-hematologic toxicity was seen, and there were no treatment breaks secondary to dermatologic or gastrointestinal toxicity [16].
Subsequently, Salama et al. published a pooled, multi-institution, retrospective analysis of 53 patients treated with IMRT and chemotherapy for anal cancer from the University of Chicago, University of Illinois, and the Mayo Clinic [17]. Median dose was 45 Gy to the pelvis and inguinal nodes and 51.5 Gy to the primary tumor and involved nodes. Fifteen percent of patients experienced grade 3 GI toxicity, and 37 % of patients experienced grade 3 dermatologic toxicity. All grade 4 toxicity was hematologic. Tumor control outcomes after IMRT were comparable to historical controls utilizing 2D or 3D techniques with 92.5 % of patients achieving complete clinical response after treatment. Since these initial reports, others have published IMRT results showing similar tumor control and colostomy-free rates comparable to historical results of studies using conventional radiation and chemotherapy. This treatment is well tolerated with favorable rates of acute dermatologic, gastrointestinal (GI), and genitourinary (GU) toxicity (Table 18.1) [18–24]. Dosimetric analyses also indicate that the use of IMRT allows for significantly lower dose to organs at risk (OARs) [25–28].
Inherently, retrospective series have limitations, especially in toxicity scoring. The most compelling data indicating that IMRT is an alternative to 2D or 3D conformal radiation therapy is from the recently published RTOG 05-29 trial. This prospective, multi-institution phase II study of 63 patients evaluated dose-painted IMRT with 5FU and MMC [29]. The primary study hypothesis was that IMRT would reduce grade 2+ GI/GU toxicity by at least 15 % compared to the 5FU/MMC arm of the RTOG 98-11 study utilizing conventional radiotherapy techniques with 5FU/MMC. The study did not show any difference with respect to the primary endpoint with grade 2+ GI/GU toxicity rates of 77 % in both RTOG 05-29 and RTOG 98-11. Importantly, the study did show lower grade 3+ GI toxicity rates (21 % vs. 36 %, p = 0.0082), lower grade 3 + dermatologic toxicity rates (23 % vs. 49 %, p < 0.0001), and lower grade 2+ hematologic toxicity rates (73 % vs. 85 %, p = 0.032). At 2 years the rates of locoregional and colostomy failure, DFS, and OS of RTOG 05-19 were similar to disease outcomes from RTOG 98-11 [30].
An additional finding of RTOG 05-29 is the importance of real-time quality assurance processes. Eighty-one percent of the cases required at least 1 planning revision on central, rapid pretreatment plan review with 46 % of cases requiring multiple resubmissions and re-reviews [29]. In 21 % of the patients, the gross tumor volume was inaccurately delineated. Errors in contouring of the elective nodal volumes were also common especially in definition of the mesorectum, presacrum, inguinal fossa, and iliac nodes in 55 %, 43 %, 33 %, and 31 % of cases, respectively. Normal structures, including small bowel and large bowel, were inappropriately delineated in 60 % and 45 % of cases, respectively. We have outlined previously published phase III randomized studies using conventional radiation as well as retrospective series and RTOG 05-29 using IMRT to allow comparison of tumor control rates, colostomy-free survival rates, and acute toxicity rates in Table 18.1.
Toxicity with chemoradiotherapy for anal canal cancer is significant. In RTOG 98-11, rates of chronic grade 3+ toxicity were 50 % with conventional radiation and 5FU/MMC [31]. Patients followed for a median of 66 months after conventional chemoradiotherapy were reported to have significant long-term impairment of health-care-related quality of life in a Norwegian study [32]. Lower rates of acute toxicity and dosimetric analyses indicate that IMRT may also decrease rates of late toxicity; however, these data are not mature. It will be important to continue to follow patients treated with IMRT closely to assess these outcomes.
18.3 Evaluation and Staging
We recommend that patients with newly diagnosed anal cancer be evaluated in a multidisciplinary setting by a surgeon, medical oncologist, and radiation oncologist with expertise in the treatment of anal cancer [33]. Patients who are being considered for radiation therapy and chemotherapy should undergo complete physical examination including thorough clinical evaluation of inguinal lymph nodes, digital rectal examination, and proctoscopy to determine tumor extent. In females, a pelvic examination to rule out vaginal extension is indicated as well as identification of synchronous gynecological cancers (HPV related). All patients should have a biopsy to confirm invasive malignancy at the primary site. In patients with suspicious inguinal adenopathy, biopsy can be performed to clarify the diagnosis as it may alter radiation treatment volumes and doses. Axial CT imaging of the chest, abdomen, and pelvis is critical for appropriate staging. Anal cancer most commonly spreads by local and lymphatic invasion: however, in about 13 % of cases, distant metastases are present at initial presentation [34]. Distant metastases are most commonly seen in the liver and lungs [35].
An alternative to CT or MRI is PET/CT, which can be useful for assessing disease extent and often assists in treatment planning. Multiple, small studies have shown that PET/CT alters staging in about 20 % of patients compared to conventional imaging due to improved sensitivity in detection of primary tumor, involved regional lymph nodes, and distant metastases [36–40]. Winton et al. reported that initial diagnostic PET/CT altered design of radiation treatment fields in 8 of 61 (13 %) of patients with anal cancer [36]. Similarly a series of 50 patients from Australia reported 19 % of cases underwent treatment planning revision based on pelvic or nodal inguinal involvement on PET/CT [39].
18.4 Patterns of Spread
An understanding of local and lymphatic patterns of invasion is critical for accurate radiation treatment planning. Approximately 50 % of patients with squamous cell carcinoma of the anal canal will invade into the rectum and/or perianal skin [41]. Careful physical exam and attention to axial imaging as described above should rule out T4 disease with local invasion into nearby structures such as the pelvic bones, prostate, vagina, bladder, or urethra.
Lymphatic involvement is common in anal cancer with a reported 31 % incidence of regional lymphatic spread at presentation [34]. Tumors arising from the anal canal, anal verge, or perianal skin can have similar lymphatic drainage as rectal malignancies due to a rich lymphatic plexus between the anal canal and rectum. Tumors below the dentate line predominately drain to the superficial inguinal and femoral nodes [42]. For distal tumors which are deeply infiltrating or poorly differentiated, the reported rate of inguinal node metastases is 63 % [2]. Tumors within the anal canal at or above the dentate line are more likely to drain to the perirectal and internal iliac lymph nodes with rare extension to the external iliac or common iliac nodes except in advanced cases [42].
18.5 Simulation
After clinical and radiological staging, CT-based simulation is performed for radiation treatment planning. If available, PET/CT at the time of simulation may be helpful to define local and regional target structures. Patients can be simulated in the supine or prone position, and there are benefits to each approach in the appropriate clinical setting. Prone setup with a false tabletop allows for improved small bowel avoidance and may be useful in individuals with a large pannus and pelvic node involvement. Supine setup is usually more reproducible with less setup variability, potentially allowing for reduced PTV margins and smaller treatment fields. We typically simulate patients for anal cancer IMRT planning in the supine position with legs slightly abducted (frog legged) with semirigid immobilization in vacuum-locked bag or alpha-cradle. Patients are instructed to maintain a full bladder for simulation and treatment. In males, the external genitalia are typically positioned inferiorly such that setup is reproducible. In females, a vaginal dilator can be placed to help delineate the genitalia and displace the vulva, anterior vagina, and urethra away from the primary tumor. A radio-opaque marker should be placed at the anal verge, and perianal skin involvement can be outlined with radio-opaque catheters. It may be helpful to place a catheter with rectal contrast in the anal canal at the time of simulation for tumor delineation. In patients with adequate renal function, IV contrast facilitates identification of the pelvic and groin vasculature (which approximates at-risk nodal regions). Oral contrast identifies small bowel as an avoidance structure during treatment planning. For tumors involving the perianal skin or superficial inguinal nodes, bolus should be placed as necessary for adequate dosing of gross disease in these areas. The routine use of bolus may not be necessary as the tangential effect of IMRT may minimize skin sparing. In situations where adequate dosing of superficial targets is uncertain, in vivo diode dosimetry with the first treatment fraction can ensure appropriate dose at the skin surface.
18.6 Target Volume Definition
Target volume definition should be performed per ICRU 50 recommendations [43]. Gross tumor volume (GTV) should include all primary tumor and involved lymph nodes, utilizing information from physical examination, endoscopic findings, diagnostic imaging, and simulation planning study for delineation. Clinical target volumes (CTV) should include the gross tumor volume plus areas at risk for microscopic spread from the primary tumor and at-risk nodal areas. If the primary tumor cannot be determined with available information (such as after local excision), the anal canal may be used as a surrogate target. Ortholan et al. [44] published a study of 181 patients with anal cancer without inguinal nodal involvement at presentation. Seventy-five patients received elective inguinal irradiation compared to 106 patients who did not receive elective inguinal irradiation. With a median follow-up of 61 months, rates of inguinal recurrence were 2 % in patients receiving inguinal irradiation compared to 16 % in patients without inguinal irradiation. Given these recurrence rates, our general policy is that pelvic and inguinal nodes should be routinely treated in all patients. When using IMRT, a separate CTV volume for each planned treatment dose tier is contoured. Our approach has been to define three tiers: a gross disease only volume, a high-risk elective nodal volume (including gross disease), and low-risk elective nodal volume (including gross disease) [18, 19]. These volumes are determined by the presence or absence of tumor based on physical exam, biopsy, diagnostic and planning studies, and risk of nodal spread depending on tumor stage at presentation. The rationale for this approach is based on the shrinking fields technique [6, 7]. At other institutions using IMRT and in RTOG 05-29, a gross disease volume with a single elective nodal volume is used to deliver the prescribed course (dose painting) [16, 20–24, 29, 45].
In defining the gross disease CTV around the primary tumor, an approximately 2.5 cm margin around GTV should be used with manual editing to avoid muscle or bone at low risk for tumor infiltration. To define the gross disease CTV around involved nodes, a 1 cm expansion should be made beyond the contoured involved lymph node with manual editing to exclude areas at low risk for tumor infiltration. Additionally, in our practice, the entire mesorectum below the level of small bowel is included within the volume defined as gross disease CTV.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree