CHAPTER 4 Analysis of Mass Effect
Lesions or processes that cause compression, distortion, and/or displacement of intracranial contents may be said to have “mass effect.” One important concept to understand is that mass effect is a manifestation on imaging of various intracranial processes (including tumor, hemorrhage, ischemia, and trauma) and not a diagnosis in itself. An analogy to clinical medicine is that vertigo is not a diagnosis in itself but rather a symptom with multiple possible underlying causes (such as posterior fossa infarct or vestibular abnormality). Detection and characterization of mass effect is a fundamental skill of neuroradiology. Accurate characterization of mass effect helps to precisely define the location of a lesion, which is crucial to forming an accurate differential diagnosis. In addition to diagnostic value, detection and prompt reporting of mass effect is also of great importance to the care of patients, especially those with life-threatening herniation. The goal of this chapter is to introduce key concepts and methods of analysis of mass effect to the beginning radiologist.
Prior to the advent of cross-sectional imaging, intracranial masses were localized and characterized by catheter angiography or other invasive techniques such as pneumoventriculography, pneumocisternography, and metrizamide ventriculography/cisternography.1,2 CT and MRI are now the modalities of choice for evaluation of intracranial space-occupying lesions. Catheter angiography is now largely reserved for further characterization of and therapy for vascular intracranial lesions (e.g., preoperative evaluation and embolization of meningioma).
Mass effect may be due to direct displacement of intracranial contents by discrete space-occupying lesions, such as benign and malignant neoplasms, non-neoplastic masses (e.g., arachnoid cyst), localized hemorrhage, or abscess. Mass effect may also be caused by brain swelling or edema. The pathophysiology of abnormal accumulation of fluid within the brain parenchyma is complex.3 Edema may be described in terms of etiology (osmotic, hydrostatic, hyperemic), microscopic location (extracellular or intracellular), or macroscopic anatomic location (e.g., gray matter vs. white matter). In simple terms based on physical location with regard to cell membranes, brain edema may be classified as vasogenic (extracellular) or cytotoxic (intracellular). Although these two descriptors of edema in reality often coexist and are not mutually exclusive, cerebral edema, on imaging, is often described as one or the other. In simple terms, cytotoxic edema demonstrates reduced diffusion (reduced apparent diffusion coefficient [ADC]) whereas vasogenic edema does not (normal or increased ADC). Cytotoxic edema is more prominently found in gray matter, whereas vasogenic edema is more prominent in white matter. The causes of cerebral edema are myriad. Examples include regional cytotoxic edema due to ischemic infarct, local or regional vasogenic edema associated with tumor or infection, post-traumatic edema, and generalized cerebral edema due to diffuse insult such as hypoxia/ischemia.4
According to the Monro-Kellie hypothesis, the intact calvaria creates a fixed intracranial space5 that under normal conditions contains (1) brain (and its meningeal coverings), (2) blood (within vessels and dural venous sinuses), and (3) cerebrospinal fluid (within the subarachnoid space and ventricles).6 Although the pathophysiology of intracranial pressure/volume relationships is indeed much more complex,7 this basic understanding of the Monro-Kellie hypothesis is sufficient for the beginning radiologist. A corollary to this hypothesis for the radiologist is that under normal conditions the intracranial contents demonstrate a clearly defined midline with bilateral symmetry. Any disruption of this normal equilibrium (e.g., a space-occupying lesion and/or edema) may change the appearance of the contents of the intracranial space.
ANATOMY
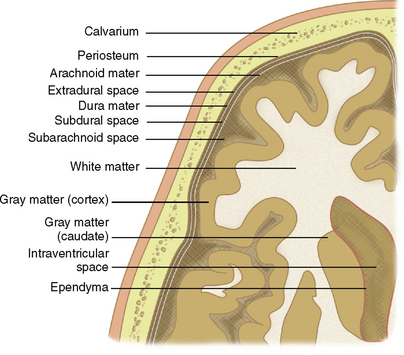
The discussion of analysis of mass effect may begin with a review of basic anatomic principles. Figure 4-1 presents a diagrammatic representation of the principles discussed here.
Brain Parenchyma: Gray and White Matter
The parenchyma of the brain may be divided into gray matter, consisting of unmyelinated neurons, and white matter, consisting of myelinated axons. The cortical mantles as well as deep nuclei of the cerebrum and cerebellum are composed of gray matter. White matter is characteristically located deep to the cortex and may be further described by location: subcortical, deep, and periventricular. The ventricles are lined by ependyma.
Meninges
The brain is held in place by the tough inelastic dura mater (also called meninx fibrosa or pachymeninx [plural, pachymeninges]), which under normal conditions is adherent to the periosteum of the inner surface of the calvaria. Reflections of the dura mater create fixed compartments within the intracranial space (Fig. 4-2). Named for its crescentic shape (L. falx, “sickle”), the falx cerebri separates the cerebral hemispheres along the interhemispheric fissure. The cerebrum and cerebellum are separated by the tentorium cerebelli (L. tentorium, “a shelter made of stretched skins”). The brain stem traverses an opening of the tentorium called the tentorial incisura (or hiatus).
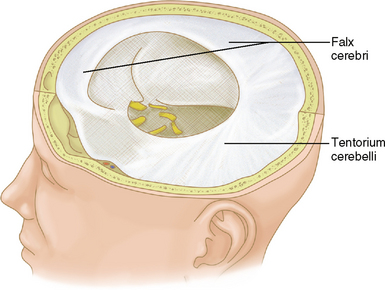
FIGURE 4-2 Gross specimen with dura retained in place to illustrate the falx cerebri and tentorium cerebelli.
(From Thibodeau GA, Patton KT. Anatomy and Physiology, 4th ed. St. Louis, Mosby, 1999, p 376.)
The delicate pia mater and arachnoid mater comprise the leptomeninges, which cover the superficial cortical surfaces of the brain. The pia mater, adherent to the brain surface, follows the convolutions of the sulci and gyri. The arachnoid mater, external to the pia mater, covers the brain surface but follows the dural contours (i.e., it does not extend into the gyri and sulci). The reticulated inner surface of the arachnoid mater has fibers that intermingle with the surface of the pia. Free-flowing cerebrospinal fluid (CSF) is found in the subarachnoid space between the arachnoid mater and pia mater. Under normal conditions, the smooth outer surface of the arachnoid mater, overlying dura mater, and calvarial periosteum are in close approximation to each other with no discernible separation (i.e., the “subdural space” and “epidural space” are collapsed potential spaces).8
Intra-axial versus Extra-axial
Clues to the extra-axial location of a tumor (Fig. 4-3) may include displacement of pial vessels subjacent to the mass, buckling of the gray matter/white matter junction, widening of the adjacent subarachnoid space, a “cleft” of CSF between the brain parenchyma and the mass, a wide base along the dural or calvarial surface, and changes within the adjacent bone such as hyperostosis associated with meningioma (Fig. 4-4) or smooth scalloping associated with epidermoid (Fig. 4-5).9,10
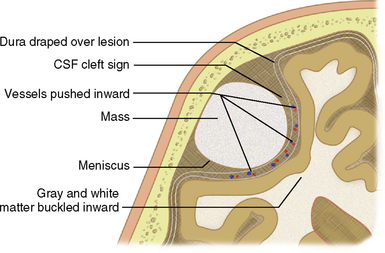
FIGURE 4-3 Clues to extra-axial location of a mass.
(From Grossman RI, Yousem DM [eds]. Neuroradiology Requisites. St. Louis, Mosby, 2004, p 275.)
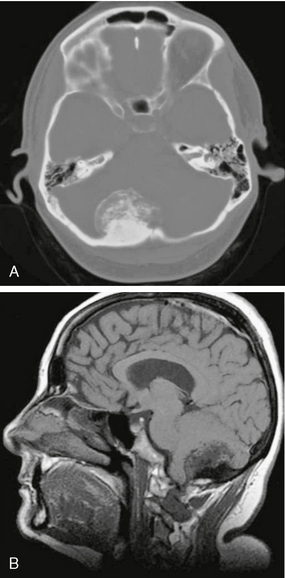
FIGURE 4-4 Posterior fossa meningioma in a 45-year-old woman with chronic headaches. A, Noncontrast CT (NCCT) demonstrates hyperostosis of the adjacent right occipital bone as well as calcification/ossification of the mass. B, Suggestion of hyperostosis and calcification (T1 lengthening) is evident on sagittal T1W precontrast MR image.
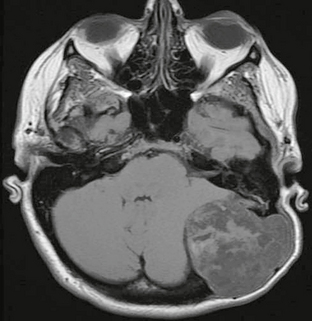
FIGURE 4-5 Posterior fossa epidermoid in a 40-year-old woman with palpable left occipital mass. Axial T1W noncontrast image demonstrates a heterogeneous extra-axial mass extending through the calvaria, with smooth scalloping of bone margins.
The subarachnoid space, between arachnoid and pia, normally contains CSF. The subarachnoid space (SAS) may be subdivided into the peripheral SAS and the basal cisterns. Subarachnoid hemorrhage may present as high density within these CSF spaces (Fig. 4-6). The subdural space, between dura and arachnoid, is normally a potential (collapsed) space. Subdural hematoma results from accumulation of blood products between the dura and arachnoid (Fig. 4-7). The dura is normally adherent to the periosteum of the inner table. Hematomas accumulating between the dura and periosteum are termed epidural or extradural (Fig. 4-8).
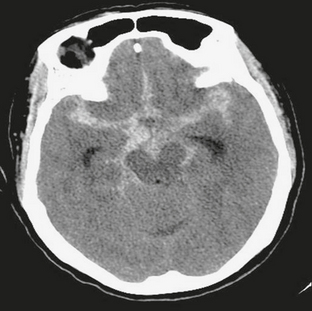
FIGURE 4-6 Acute subarachnoid hemorrhage in a 61-year-old woman with acute onset of headache and neck pain. NCCT demonstrates high density within the subarachnoid space, generalized cerebral edema with effacement of sulci, and early hydrocephalus with prominence of the temporal horns.
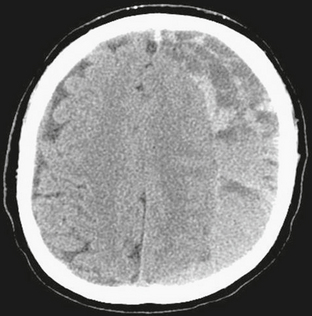
FIGURE 4-7 Subdural hematoma in a 63-year-old man with altered mental status. NCCT demonstrates left holohemispheric crescentic mixed density fluid collection with compression and displacement of subjacent brain and effacement of sulci. On the right there is minimal dural thickening, but the dura is closely approximated to the calvarial periosteum.
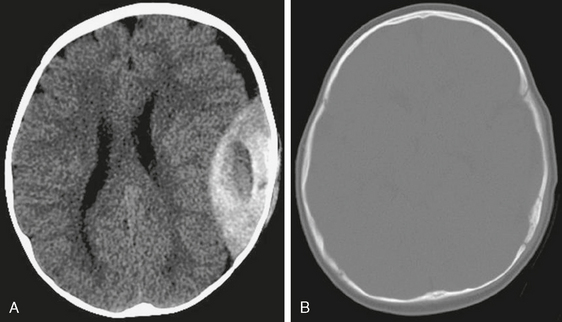
FIGURE 4-8 Acute epidural hematoma in a 6-month-old male infant with vomiting after a fall from a bed. A and B, NCCT of the brain reveals biconvex hyperdense hematoma with central low density overlying a subtle nondisplaced fracture of the squamous portion of the left temporal bone. Note normal appearance of the patent coronal and lambdoid sutures.
The intraventricular space (IVS) may also be considered a subdivision of the extra-axial compartment. The IVS contains CSF and choroid plexus and is lined by ependyma (in contrast to the peripheral SAS, which is lined by pia and arachnoid). Figure 4-9 demonstrates an intraventricular meningioma. CSF communicates between the IVS and SAS via the foramen of Luschka and choroid fissures.
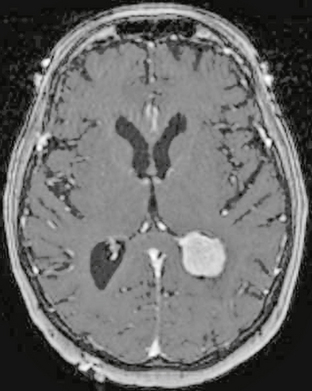
FIGURE 4-9 Intraventricular meningioma in a 73-year-old woman with headache. Preoperative 3D FSPGR post-gadolinium MR image demonstrates an enhancing intraventricular mass, with subtle expansion of the trigone of the left lateral ventricle. Note subtle anterior displacement and enhancement of the choroid plexus.