Fig. 1
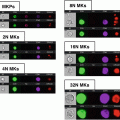
The similarity histogram and a representative imagery panel of ARPE-19 cells non-stimulated (a, b) and stimulated with 10 ng/ml TNF-alpha (c, d). Ch2—Bright-field; Ch3—staining with NF-kappa Beta antibodies, conjugated with FITC; Ch6—DRAQ5 staining; Similarity score for unstimulated ARPE-19 cells equals 0.33 and for stimulated with TNF-alpha equals 2.27
2.
Apply Compensation wizard under IDEAS software in order to create compensation matrix. For this purpose, use single controls (raw image files or .rif files) acquired in the absence of bright-field illumination. For example, we created single controls for cells stained only with nuclear dye and for cells, stained with antibody against translocated molecule (NF-kappaB). Once the compensation matrix process is finished, IDEAS software will perform corrections on the imagery and apply calculated compensation values to raw files creating new compensated image files (file extension .cif). The IDEAS application then calculate features values as specified by created template that can be saved as data analysis files (file extension .daf). The compensation template can be saved for future use as .ctm file. Details of the spectral compensation sequence are described in the manual for ImageStream (available for internet downloading as IDEAS version and from company website).
3.
Next, use the Nuclear Translocation wizard or perform the analysis of experimental .daf files. Alternatively, perform next steps to analyze the experimental data: identify single cells based on nuclear imagery and staining with DRAQ5; exclude out-of-focus events; select positively stained subpopulation for the translocated molecule (NF-kappaB) and DRAQ5.
4.
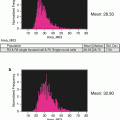
Using IDEAS analytical software feature (“Similarity” feature) calculate similarity scores for cells based on a logarithmic transformation of Pearson’s correlation coefficient [21]. The user defines the gate on fluorescence intensity histogram of cell population stained with antibodies against translocated protein and analogous histograms of negative and positive controls. Cells with low similarity score correspond to predominantly cytoplasmic distribution of NF-kappaB, whereas the higher similarity score defines higher level of translocation. The difference (shift) between stimulated and not stimulated cell populations can be evaluated with nonparametric statistical criteria such as Mann–Whitney test.
4 Notes
1.
Cells for NF-kappaB translocation can be activated with a number of stimuli, such as LPS, TNF-alpha, phorbol esters, and interleukin-1. Preliminary titration of LPS may require. TNF-alpha can be used in the physiological range (0.2–1.5 ng/ml) [22] as well as in the supra-physiological range (higher than 1.5 ng/ml) [23, 24].
We purchased lipopolysaccharide (LPS) from E. coli or S. typhimurium from Sigma (St. Louis, MO, USA). Other activators for NF-kappaB, namely TNF-alpha, phorbol esters, interleukin-1α (IL-1α), and IL-1β were purchased from R&D Systems (Minneapolis, MN).
2.
The polyclonal and monoclonal antibodies from different companies should be checked by flow cytometry in the beginning of the protocol in order to identify a correct antibody and, perhaps, a correct clone which will show significant increase in a fluorescence after activation of cells with LPS or TNF-alpha. Choosing the right antibody clone is critical, since translocation may involve a conformational change of the epitopes of translocated molecule.
3.
We use small molecular weight fluorochromes for antibody detecting the translocated molecule (FITC, Alexa 488, Alexa 647, Cy5, and other small molecules from Alexa and cyanine families). Large proteins, such as multi-subunit R-phycoerythrin (PE) (M.W. 240 KDa) and PE-based tandem dyes have difficulties to traverse inside nuclei. The fluorochromes sensitive to fixation and permeabilization should be also excluded (such as Pacific Blue fluorochrome, tandem dyes, etc.). It is recommended to determine the stability of fluorescent signal after fixation.
4.
High concentrations of PFA (4 % and such) in the fixation buffer can diminish a fluorescence of some fluorochromes since it has an effect on conformational protein epitopes. Compatibility of fixation buffer with translocated molecule should be evaluated in the preliminary experiments.
5.
Permeabilization buffer is critical for successful intracellular staining and proper visualization of nuclear translocation. With a variety of available reagents we recommend to check a few variants of commercial buffers and choose the best suitable for the antibody against NF-kappaB molecule. In house prepared buffers also can be used.
6.
Significant increase in NF-kappaB translocation can be seen after 20 min after simultaneous stimulation with both LPS and TNF-alpha.
7.
Importantly, to use for centrifugation siliconized plastic tubes. Siliconization involves placing a thin layer of siliconizing solution (dimethyldichlorosilane) or Sigmacote (Sigma-Aldrich, St Louis) onto glass or plastic surfaces to make them extremely hydrophobic. “Low-adhesion” plastic is not a good replacement for siliconized tubes. Siliconized tubes can be purchased or prepared by short-time incubation with Sigmacote. Shortly, fill a centrifuge tube with Sigmacote all the way to the top. Collect the Sigmacote from the tube (with glass Pasteur pippete) and transfer back to the original bottle or pour in the next tube. Rinse each time with ddH2O and let it completely dry. The adequate siliconization is retained by plastic and glass for a long time (years).
8.
Blocking step is important. The sequence for staining events with anti-mouse antibodies would include next steps: incubating cells with anti-Fc antibodies for 30 min on ice; a quick spin to remove anti-Fc-antibodies supernatant; staining for surface antigens; fixation and permeabilization for intracellular staining. The selection of blocking serum for antibodies originated from other species (rat, rabbit, others) depends on the species of used antibodies. Generally, blocking with serum from the same animal species as a secondary antibody is best. The alternative is to use 1–3 % of bovine serum albumin (BSA). Optimization of blocking conditions is necessary in order to decrease background of staining on images.
9.




Titration of antibodies (primary antibodies and secondary conjugates) and nuclear dyes is necessary in order to prevent saturation of images. Staining volume is very important and titration of antibodies should be performed in the same volume as sub sequential staining. Shortly, start at 1:50 dilution and dilute by twofold steps. Incubate with primary antibody at least 60 min at room temperature. Longer incubation times may be required for some antibodies, such as overnight incubation at 4 °C. Verify the staining with antibodies of different cellular compartments by fluorescent microscopy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree