Fig. 1
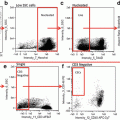
Granulocyte Gating. Using the entire cell population, focused cells are identified using a histogram of bright-field gradient RMS (a), followed by single cells that are identified using a dot plot of bright-field aspect ratio vs. bright-field area (b), and finally granulocytes (CD45+/66b+) are identified using a dot plot of CD66b vs. CD45. This gating pattern results in the positive identification of at least 3000 granulocytes per patient sample
3.
Save template and using the batch-processing wizard, apply the negative control template to the 10, 20, and 40 min incubations for the same sample.
4.
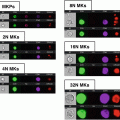
Open the 40 min *.cif file and create a dot plot of bright detail intensity for bioparticles (x-axis) vs. bright detail intensity for oxidative burst (DHE, y-axis) to identify subsets of activated granulocytes (see Note 21 ) (Fig. 2).
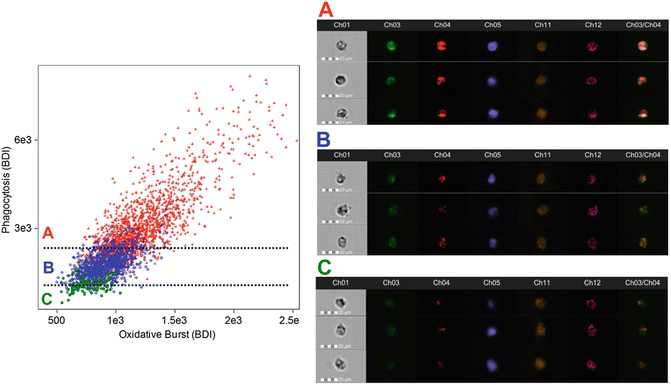

Fig. 2
Activated Granulocyte Subset Gating. After the identification of granulocytes using cell-surface markers (CD45+/66b+), a daughter plot is generated of bright detail intensity for phagocytosis vs. bright detail intensity for oxidative burst. From this plot it is possible to identify and quantify high-active (red, a), moderate-active (blue, b), and low-active (green, c) granulocytes. Additional analysis reveals that with each activation subset there are changes in the co-localization of phagocytosis and oxidative burst signals (the ch3/ch4 merge image demonstrates this effect). High-active granulocytes had the greatest co-localization, while low-active granulocytes had the least co-localization
4 Notes
1.
Aliquot volume should be chosen based on the number of samples that are expect to be analyzed on a given day. In our laboratory, we typically analyze a minimum of six blood samples per day. Thus, we froze enough bioparticles for 24 assay tubes plus an extra 10 % (aliquot volume = 528 μL).
2.
Care should be taken to not expose bioparticles to more than one freeze–thaw cycle. Any unused bioparticles should be discarded or held in the refrigerator. Any bioparticles stored in the refrigerator should be used within 7 days of thawing.
3.
Similar to bioparticles, this should be aliquoted and frozen in increments that match the targeted experimental outcomes. Any unused DHE stock should be discarded each day. As with any fluorescent reagent, titration will be needed to confirm positive staining. The concentration suggested in this method should be considered a starting point that will need to be refined in each laboratory situation.
4.
N-ethylmaleimide does not easily dissolve in solution. For this reason, it may be necessary to place the solution in the 37 °C bead bath. When freezing aliquots, identify a volume that matches the quantity of bioparticles and DHE that you have frozen separately.
5.
When thawing N-ethylmaleimide, it should be done as quickly as possible and the thawed aliquot should be kept in the 37 °C bead bath until addition to the assay. Failure to keep the solution heated will result in the N-ethylmaleimide falling out of solution. If this occurs, you should discard that aliquot and thaw a new aliquot using the same procedure.
6.
CD66b and CD45 antibodies were titered in a series of experiments completed prior to this method. It is necessary to titer antibodies in your laboratory and on your flow cytometer prior to completing testing of this nature. Additional cell-surface (i.e., CD197 etc.) can be included in the staining panel to assess other aspects of granulocyte function.
7.
It is important that aliquots of 7-AAD be stored in a light sensitive (i.e., dark colored) tube. This can also be accomplished by wrapping a 1.5 mL centrifuge tube in aluminum foil.
8.
All blood collection procedures described in this method were conducted in accordance with the Declaration of Helsinki and approved by the UNT institutional review board (IRB) for human subjects. All subjects gave written consent for blood collection, which was used in the present method to ensure that they were apparently healthy, of normal body weight, and disease free.
9.




It is important to make sure that collected blood is mixed gently by inversion a minimum of ten times.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree