Fig. 1
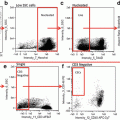
Initial processing of IFC MK panel files and merging of CD41+ events. (a) Determination of enucleated red blood cells versus nucleated cells by DRAQ5 signal intensity. This strategy is used to record the number of nucleated cells in each MK panel file as well as to record the frequency of nucleated cells in the unmanipulated DRAQ5-only file for each biologic sample. This population contains all collected events, including multicellular events. For more distinct 2N and 4N ploidy peaks, single cells can be gated first with the IDEAS “single-cell” building block. (b) Events containing CD41 positivity are gated based on CD41 signal intensity. For this sample, 2 % of the 50,000 collected events fall into the CD41+ gate, which is set based on a combined isotype/flour minus one (FMO) control
2.
For each 50,000-event MK panel file, gate events with CD41 positivity (Fig. 1b) and create a CIF file from this CD41+ population.
3.
Merge the CD41+ CIF files from all MK panel files for a given sample into a single CD41+ file. Sum the nucleated cells for the original 50,000-event files that contributed to the merged file (from step 3.4.1) to obtain total nucleated cells.
4.
Figure 2 details the sequential gating strategy to determine the number of MKs from the merged CD41+ file. Overall, the gates are set based on MK yield rather than purity, and purity is achieved with nested gating. The percentage of parent population cells that fall within each gate for this sample is indicated in Fig. 2; however, it is important to visualize the cells at gate boundaries and to adjust these gates to include all MKs (Fig. 3) (see Notes 25 and 26 ).
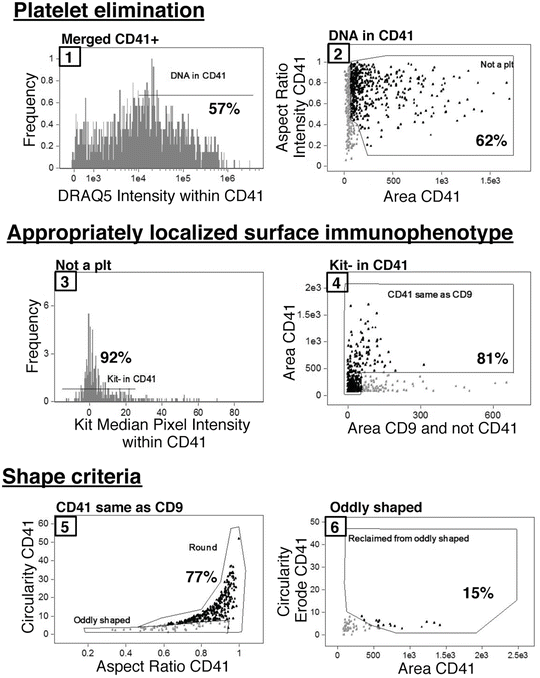
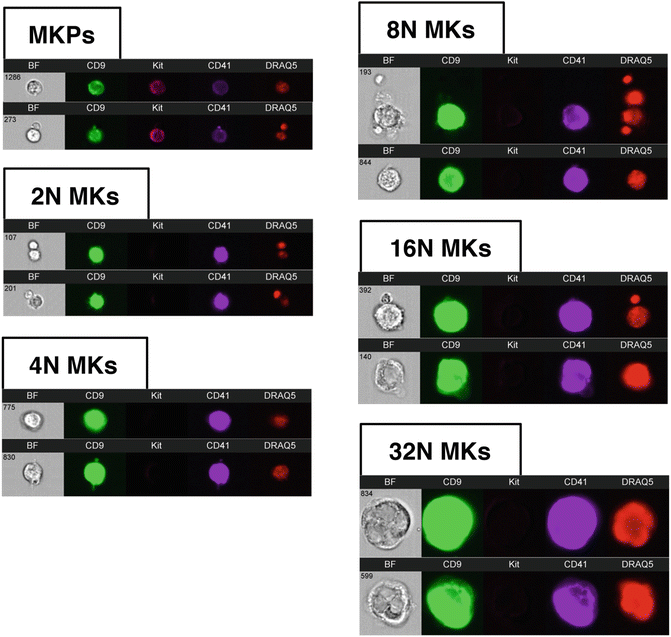

Fig. 2
Nested IFC gating strategy for MKs beginning with merged CD41+ populations. Gates are numbered sequentially and the percentage of the parent population that falls within a given gate is indicated for this sample. First, CD41+ platelets attached to non-MK nucleated cells are eliminated from analysis based on a lack of DRAQ5 signal intensity within the CD41 mask (1) as well as small area and irregular shape (2). The MK population is further refined by both surface immunophenotype, and appropriate localization of immunophenotypic markers with CD41 within an event. MKs are negative for Kit signal within the CD41 mask (3) and contain overlapping CD9 and CD41 signal areas (4). Additionally, cells positive for Kit signal within the CD41 mask can be further immunophenotypically refined with the strategy by Pronk et al. [13] to analyze MKPs. Finally, events that make it through the four above-described gates must fulfill shape-based criteria, which eliminate cytoplasmic fragment debris. The final MK population consists of mostly cells in the “Round” gate (5) with a few MKs with cytoplasmic extensions that are originally classified as “oddly shaped” (5) but are reclaimed based on the circularity of an eroded CD41 mask (6). The axis names have been changed for clarity; however, full descriptive names of features and masks can be found in Table 1
Table 1
Mask/feature combinations used in IFC MK lineage gating strategy
Axis label | Default IDEAS mask/feature nomenclature |
|---|---|
DRAQ5 intensity | Intensity_Morphology(M11,Ch11)_Ch11 |
CD41 intensity | Intensity_Object(M07,Ch07,Tight)_Ch07 |
DRAQ5 intensity within CD41 | Intensity_Object(M07,Ch07,Tight)_Ch11 |
Aspect ratio intensity CD41 | Aspect Ratio Intensity_Object(M07,Ch07,Tight) |
Area CD41 | Area_Object(M07,Ch07,Tight) |
Kit median pixel intensity within CD41 | Median Pixel_Object(M07,Ch07,Tight)_Ch06 |
Area CD9 and not CD41 | Area_Object(M02,Ch02,Tight) and not Object(M07,Ch07,Tight) |
Aspect ratio CD41 | Aspect Ratio_Object(M07,Ch07,Tight) |
Circularity CD41 | Circularity_Object(M07,Ch07,Tight) |
Circularity erode CD41 | Circularity_Erode(M07,7) |

Fig. 3
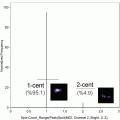
Examples of MK lineage cells by IFC. Immunophenotypically defined MK progenitors can be analyzed from the Kit + population (Fig. 2, plot 2, ungated population). Examples of MKs in ploidy classes ranging from 2N to 32N are shown. Images from left to right for each event row are Brightfield (BF), CD9, Kit, CD41, and DRAQ5. All MK images in this figure were obtained with identical display settings, with the exception of MKP images which have been adjusted to optimize viewing. Note that localization of signal measurements to the CD41 mask in the gating strategy presented in Fig. 2 allows for the analysis of MKs even when physically associated with other nucleated cells in an event (top 8N and 16N event examples)
5.
6.
Calculate MKs/femur: [MKs/merged CD41+ file (step 3.4.5)] × [sum of total nucleated cells/merged CD41+ file (steps 3.4.1 and 3)] × [frequency of nucleated cells (step 3.4.1 DRAQ5-only file)] × [total cells/femur (step 3.1.7)] (see Note 27 ).
7.
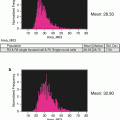
In addition to the quantification of MK frequency in murine bone marrow, this gating strategy generates a primary MK population that can be further studied by restricting analyses to the CD41 mask. For example, we can divide the MK population into ploidy classes by DNA content, which is an important parameter of endomitotic progression through the maturing MK compartment. This is achieved by measuring only the DRAQ5 signal within the CD41 mask for each event (as in Fig. 2 #1) (see Notes 28 and 29 ). While the ploidy peaks may not be discrete given the relatively small number of MKs sampled by IFC compared to traditional flow cytometry, we circumvent this issue by identifying the 2N and 4N peaks for total DRAQ5 intensity in the original 50,000-event file (Fig. 1a) and use these values to set the 2N and 4N gates. The subsequent ploidy class gates (8N, 16N, 32N) can then be determined based on DRAQ5 intensity doubling. Examples of MKs in each ploidy class are provided in Fig. 3.
4 Notes
1.
We utilize PB2 buffer for dissection and washing. PB2 was optimized for the preservation of erythroblast precursors for flow cytometric analyses [11]. Phosphate-buffered saline can be substituted.
2.
We routinely use a 40 μm cell strainer to remove large unseparated cell clumps from titurated marrow cell suspensions. While we retain large polyploid MKs, these cells have been reported to be capable of reaching over 50 μm in diameter in the mouse [12]. Therefore, a larger pore size (100 μm) may be advisable for certain experiments.
3.
We use normal rat serum because all of our primary antibodies are produced in rat. Species selection for blocking buffer serum will depend on the species of antibodies used.
4.




Anti-CD41 antibodies are commercially available conjugated to a large number of fluors. We prefer to use eFluor450-CD41, which is collected in channel 7, as there is very little signal overlap from other channels into channel 7. Accordingly, for experiments aimed at obtaining many MKs for analysis, a cell classifier can be set to limit event acquisition based on CD41/channel 7 signal intensity even though data acquisition occurs prior to spectral compensation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree