div epub:type=”chapter” role=”doc-chapter”>
Atlas and Anatomy of PET/CT
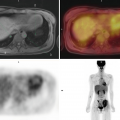
PET/CT is a combined system of positron emission tomography (PET) and computed tomography (CT) scanners. PET can detect abnormal metabolic activity in organs or lesions even before they show morphological changes and CT enables precise localization, so co-registration of functional and anatomic information is achieved in the same study, obtained on the same scanner [1]. This technology, available now for at least 10 years, has allowed great advances especially in the field of oncology, and every day it opens more fields to explore in this and other multiple pathologies [2]. Nowadays there is also the possibility of carrying out total body PET studies, which encompasses the entire body within the field of view of the scanner, allowing imaging of all the tissues and organs simultaneously. The increase in geometric coverage of total body PET and multiple adjusted parameters make the whole-body image a very sensitive study with major implications for medical imaging.
PET/CT is currently widely available in the world and many guidelines already recommend it as part of the diagnosis, staging, follow-up, or re-evaluation of various pathologies. As for PET/MR, nonspecific 18F-FDG is the most widely used an available radiotracer for PET/CT studies, and thus many other new tracers are available or under investigation, to offer better possibilities to patients and not only in the field of oncology [2, 3]. Indications for FDG PET/CT are continuously evolving according to the advances that current research allows; however, in oncology it can be useful in various stages of the disease depending on the pathology: initial diagnosis, staging, therapeutic approach, evaluation of response to treatment and recurrence. A great example, and perhaps one of the most frequently used with multiple indications is lymphoma [3, 4]. Other frequent scenarios in which it has a diagnostic utility are the evaluation of a solitary pulmonary nodule, multiple myeloma, and search for a primary tumor of unknown origin [5–7]. Its usefulness in staging due to the ability to evaluate the whole body, detect lymph node or distant metastasis, and offer some prognostic information can be extended to almost all cancer pathology, but it has been evaluated with better results in melanoma, head and neck, lung, colorectal, gynecological and esophageal cancers among others, as well as in bone and soft tissue sarcomas [3, 8–11]. Likewise, in some cases such as lung, esophageal, and colorectal cancers, it has shown great utility in radiotherapy planning with excellent results [12, 13]. Response assessment utility depends on the characteristics of the primary tumor and stage, but in lung, esophageal, and colorectal cancers, the results have been promising [10, 13, 14]. Finally, in the evaluation of recurrence, its diagnostic accuracy may also be subject to the characteristics of the primary tumor, but since a large part of the metastases are more aggressive and metabolically active, it represents a good tool for almost all tumors, especially for head and neck, lung, gynecological, and bone and soft tissue tumors. It is important to bear in mind that although it is not indicated for the diagnosis or staging of a series of non-FDG avid tumors (stomach, pancreas, hepatoma, genitourinary tract, ovary, among others), when there is a suspicion of relapse it may play an important role [3, 15, 16].
As in PET/MRI, the availability of multiple novel radiotracers has allowed great advances, especially in neuroendocrine tumors, prostate cancer, and neuro-oncology and degenerative diseases, having the same tracers previously described available for PET/CT studies [17].
In this chapter, we present multiple demonstrative examples of the different uses of FDG and non-FDG PET/CT, with the most relevant anatomical references for each case.
1 18F-FDG PET/CT
1.1 Head and Neck
1.1.1 Case 1

1. Left superior frontal gyrus
2. Left middle frontal gyrus
3. Calcified meningioma in the corpus callosum
4. Perilesional edema in the posterior left periventricular area
5. Falx cerebri
6. Right corona radiata
7. Right lateral ventricle
8. Superior sagittal sinus

1. Falx cerebri
2. Left medial frontal gyrus
3. Left caudate nucleus head
4. Perilesional edema in the posterior left periventricular area
5. Right lateral ventricle, anterior horn
6. Right putamen
7. Right cistern of lateral cerebral fossa (insular cistern)
8. Right thalamus
9. Right lateral ventricle, posterior horn
10. Straight sinus
11. Superior sagittal sinus
1.1.2 Case 2

1. Right parietalcortex, precentral gyrus
2. Hypometabolic metastasis in left parietal cortex
3. Right frontal cortex, superior frontal gyrus
4. Diffuse hypometabolism in the left parietal cortex and white matter representing perilesional edema
5. Right cingulate gyrus
6. Occipital cortex
7. Left centrum semiovale
8. Right frontal lobe
9. Right frontal skull
10. Lateral ventricles
11. Left parietal lobe
12. Left frontal scalp
13. Head of caudate nucleus
14. Left temporal lobe
15. Right occipital lobe
16. Sella turcica
17. Right auricle
18. Right temporal muscle
19. Frontal sinuses
20. Left sphenoid bone
21. Left mastoid air cells
1.1.3 Case 3

1. Suprasellar germinoma
2. Pineal germinoma
3. Lacrimal glands with mild increased activity
4. Olfactory cortex
5. Right mastoid air cells
6. Midbrain
7. Torcula Herophili (confluence of the sinuses)
8. Left middle cerebral artery
9. Ethmoid air cells
10. Left thalamus
11. Body of the left lateral ventricle
12. Anterior horn of the right lateral ventricle
13. Third ventricle
14. Posterior limb of the right internal capsule
15. Posterior horn of the right lateral ventricle
16. Frontal sinuses
17. Left eye lens
18. Left eye vitreous chamber
19. Left optic nerve
20. Choroid plexus at the right lateral ventricle
1.1.4 Case 4

1. Clival chordomas
2. Optic nerves
3. Right zygomatic bone
4. Right temporal lobe
5. Right mastoid air cells
6. Right cerebellum
7. Nasal septum
8. Right nasal bone
9. Right ethmoid sinus
10. Right sphenoid bone
11. Right internal carotid artery
12. Right petrous pyramid
13. Physiologic increased uptake at the visual cortex
14. External occipital protuberance
15. Cerebellum
16. Brainstem
17. Atlantoaxial joint
18. Soft palate
19. Nasal bones
20. Middle nasal concha
21. Hard palate
22. C2, odontoid process (dens)
23. Foramen magnum
24. Clivus
25. Sella turcica/pituitary gland
1.1.5 Case 5

1. Submandibular glands
2. Metabolically active glottis squamous cell carcinoma
3. Hyoid bone
4. Cervical transverse foramen, vertebral artery
5. Cervical vertebral body posterior arch
6. Arytenoid cartilages
7. Cricoid cartilage
8. Pre-epiglottic fat
9. Left pyriform sinus
10. Cervical vertebrae, spinous process
11. Thyroid cartilage
12. Cervical vertebral body
13. Left vocal cord
14. Left splenius capitis muscle
15. Left levator scapula muscle
16. Left trapezius muscle
17. Left parietal lobe
18. Left temporal lobe
19. Sphenoid sinus
20. Right mandible ramus
21. Sternum
22. Left parapharyngeal space
23. Left clavicle
24. Left humerus
25. Occipital lobe
26. Cerebellum
27. Cervical spine
28. Frontal sinus
29. Ethmoid cells
30. Nasopharynx
31. Oropharynx
32. Tongue
33. Epiglottis
34. Glottis
35. Trachea
1.1.6 Case 6

1. Left optic nerve
2. Right thyroid lobe, lymphoma involvement
3. Left thyroid lobe, lymphoma involvement
4. Thyroid isthmus
5. Heart, left ventricular wall uptake
6. Right subclavian vein
7. Right brachiocephalic vein
8. Trachea
9. Normal left axillary lymph nodes
10. Right humeral head
11. Left acromion
12. Right glenoid
13. Left scapular spine
14. Left clavicle
15. Left interpectoral area
16. Posterior cervical fat
17. Left pulmonary apex
1.1.7 Case 7

1. Metabolically active primary tumor in the right tonsil
2. Metabolically active metastatic lymph nodes in the right neck, level II
3. Mild hypermetabolic lymph nodes in the right lung hilum, inflammatory
4. Right renal pelvis
5. Mandible
6. Right sublingual space
7. Genioglossus muscle
8. Left masseter muscle
9. Left mylohyoid muscle
10. Left parapharyngeal space
11. Left parotid gland
12. Left oblique capitis muscle
13. Left splenius capitis muscle
14. Right mylohyoid muscle
15. Oropharynx
16. Left submandibular gland
17. Left vertebral foramen
18. Spinal canal
1.2 Chest
1.2.1 Case 1
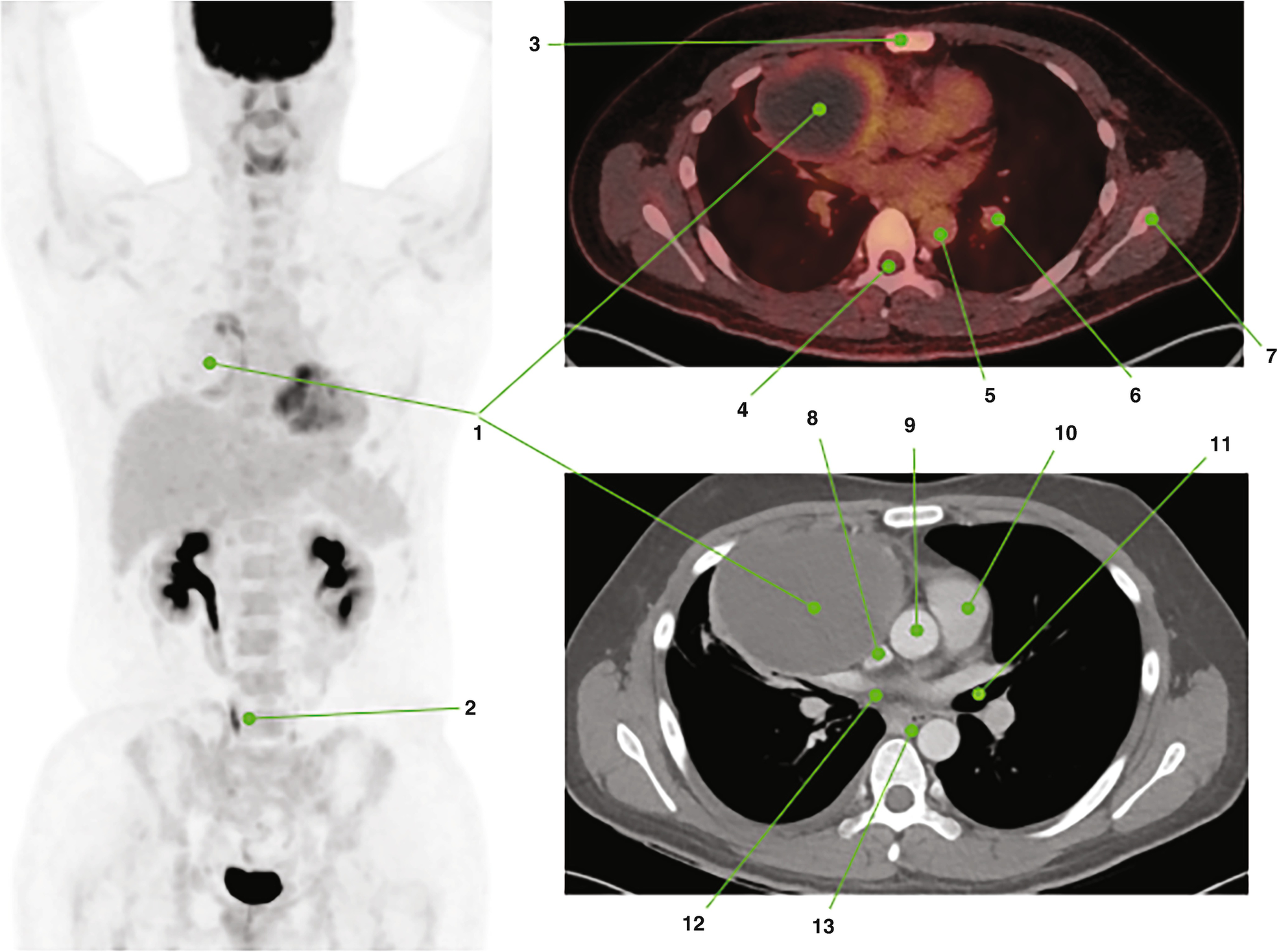
1. Primary tumor with large cystic-necrotic component and hypermetabolic solid peripheral component
2. Right ureter
3. Sternum
4. Spinal canal
5. Descending aorta
6. Left pulmonary artery, upper lobe branch
7. Left scapula
8. Superior vena cava
9. Ascending aorta
10. Main pulmonary artery
11. Left main bronchus
12. Right atrium, superior aspect
13. Esophagus
1.2.2 Case 2

1. Anterior mediastinal mass with mild homogeneous FDG uptake, consistent with thymoma type AB
2. Left ventricle
3. Left renal pelvis
4. Right middle ureter
5. Cavo-atrial junction
6. Ascending aorta
7. Descending aorta
8. Left main bronchus
9. Homogeneous cystic lesion in the right thyroid lobe
10. Left thyroid lobe
11. Aortic root
12. Right ventricle
13. Right atrium
14. Left pericardial recess
1.2.3 Case 3

1. Slight hypermetabolic anterior mediastinal mass corresponding to thymoma type B1
2. Thymoma type B1, left superior aspect with dystrophic calcifications
3. Superior vena cava
4. Common pulmonary artery
5. Extensive left pleural invasion
6. Thymoma type B1, right inferior aspect
7. Left ventricular wall
8. Right main pulmonary artery
9. Right lung lower lobe
10. Left main bronchus
11. Sternum
12. Descending aorta
13. Right internal mammary vessels
14. Pericardial fat
1.2.4 Case 4

1. Increased activity in the infiltrating mass, consistent with thymoma type B3
2. Metabolically active right lower lobe pleural seeding
3. Gastric antrum physiologic FDG uptake
4. Right upper lobe
5. Ascending aorta
6. Common pulmonary artery
7. Right lower lobe
8. Right main bronchus
9. Descending aorta
10. Left main bronchus
11. Left upper lobe
12. Left ventricle
13. Lower esophagus
14. Right ventricle
15. Right atrium
16. Left lower lobe
1.2.5 Case 5

1. Metabolically active mediastinal lymph nodes, lambda sign
2. Ascending aorta
3. Right hilar lymph nodes, level 10R
4. Right main bronchus
5. Subcarinal lymph nodes, level 7
6. Main pulmonary artery
7. Left hilar lymph nodes, 10L
8. Esophagus
9. Descending aorta
10. Left main bronchus
11. Right ureter, distal third
12. Gluteus medius muscles
13. Increased activity at left iliac bone involvement
14. Left iliopsoas muscle
15. Left iliac wing
16. Left sacroiliac joint
17. Increased activity at left gluteal soft tissue involvement
18. Right gluteus maximus muscle
19. Sacrum
20. Left acetabular roof
21. Metabolically active intergluteal lymph node
1.2.6 Case 6

1. Metabolically active tumor in the pulmonary artery
2. Angiosarcoma involvement at the main pulmonary artery
3. Angiosarcoma involvement at the right pulmonary artery
4. Angiosarcoma involvement at the right upper lobe artery
5. Ascending aorta
6. Left atrium superior aspect
7. Descending aorta
8. Right interlobar artery
9. Superior vena cava
10. Left pulmonary artery
11. Right main bronchus
12. Sternum
13. Spinal canal
14. Left rib posterior arc
15. Left main bronchus
16. Left upper lobe pulmonary artery
17. Thoracic vertebra left transverse process
18. Esophagus
19. Right costovertebral joint
1.2.7 Case 7

1. Metabolically active bulky anterior mediastinal mass
2. Hypermetabolic enlarged lymph nodes in the bilateral lower neck
3. Focal increased activity in right cardiophrenic lymph node
4. Focal increased activity in left retroperitoneal lymph node
5. Focal increased activity in right hilar lymph node
6. Thoracic vertebral body spinous process
7. Thoracic vertebral body left transverse process
8. Left scapula
9. Right ventricle
10. Right costovertebral junction
11. Descending aorta
12. Left ventricle
13. Posttreatment residual calcification
14. Residual soft tissue lesion in the anterior mediastinum with no definite FDG uptake (Deauville 1)
15. Sternum
16. Left breast tissue
17. Left major pectoralis muscle
18. Left minor pectoralis muscle
19. Left axillary fossa
20. Left rib, lateral arc
21. Carina
22. Superior vena cava
23. Esophagus
1.2.8 Case 8

1. Hypermetabolic diffuse infiltrative left breast cancer
2. Multiple hypermetabolic lymph node metastasis
3. Normal right breast tissue with mild, diffuse FDG uptake
4. Right internal mammary chain, normal
5. Sternum
6. Right atrium
7. Left atrium
8. Common pulmonary artery
9. Hypermetabolic infracarinal lymph node (level 7)
10. Ascending aorta
11. Right hilum (11R metastasis)
12. Lower esophagus
13. Left hypermetabolic lymph node (level L11)
14. Left main bronchus
15. Left scapula

1. Bilateral superior mediastinal lymph nodes (level 1–2)
2. Right lung, upper lobe
3. Level III hypermetabolic lymph node
4. Level II hypermetabolic lymph nodes
5. Level I hypermetabolic lymph node
6. Left major pectoralis muscle
7. Left minor pectoralis muscle
8. Interpectoral lymph node (Rotter lymph node)
9. Right clavicle
10. Left lung, upper lobe
11. Trachea
12. Right thyroid lobe with mild, diffuse uptake
13. Hypermetabolic left supraclavicular lymph nodes
14. Left deltoid muscle
15. Left trapezius muscle
1.2.9 Case 9

1. Metabolically active multifocal left breast cancer
2. Multiple hypermetabolic metastatic lymph nodes
3. Left renal pelvis
4. Right ureter
5. Left adnexal physiologic activity
6. Normal right breast tissue
7. Increased activity at skin thickening in the left breast
8. Mediastinal vessels
9. Left internal mammary lymph node metastasis
10. Minor pectoralis muscle
11. Metastatic left axillary lymph nodes, level I
12. Left scapula
13. Right axillary fossa
14. Trachea
15. Right subclavian vessels
16. Right lung apex
17. Left first rib, anterior arc
18. Interpectoral lymph node (Rotter) metastasis
19. Left major pectoralis muscle
20. Left second rib, posterior arc
1.2.10 Case 10

1. Metabolically active right hilar lymph nodes
2. Diffusely increased activity in ground-glass opacities at the right middle lobe
3. Left renal pelvis
4. Thin-walled cystic metastasis with peripheral uptake at the right upper lobe
5. Hypermetabolic subcarinal lymph nodes
6. Right lower lobe with multiple thin-walled cystic metastasis
7. Small left upper lobe pneumothorax chamber
8. Hypermetabolic left hilar lymph node
9. Left lower lobe
10. Diffusely increased uptake in the left ventricular wall
11. Thin-walled cystic metastasis with peripheral uptake at the left lower lobe
1.2.11 Case 11

1. Right upper lobe hypermetabolic cavitated nodule
2. Mediastinal lymph node conglomerate with uneven uptake due to necrosis
3. Trachea
4. Aortic arch
5. Right upper lobe hypermetabolic solid nodule
6. Left upper lobe metastatic ground glass nodule
7. Solid metastases
8. Right upper lobe
9. Left upper lobe
10. Right main bronchus
11. Carina
12. Descending aorta
13. Left bronchus
14. Right middle lobe
15. Anterior junction line of the pleura
16. Left lower lobe, lingula
17. Diaphragm, liver dome
18. Right ventricle
19. Left ventricle
20. Right lower lobe
21. Left lower lobe
22. Right neck level II lymph node metastasis
1.2.12 Case 12

1. Lymphoma involvement at lower cervical lymph nodes
2. Metabolically active left hilar mass, consistent with diffuse large B-cell lymphoma
3. Superior vena cava
4. Carina
5. Tip of the right scapula
6. Lymphoma involvement at upper aortocaval lymph node
7. Right breast fibroglandular tissue
8. Sternum
9. Lymphoma involvement at subcarinal lymph nodes
10. Lymphoma involvement at right hilar lymph nodes
11. Thoracic vertebral body
12. Lymphoma involvement at prevascular lymph nodes
13. Azygos vein
14. Ascending aorta
15. Esophagus
16. Descending aorta
17. Right main pulmonary artery
18. Common pulmonary artery
19. Left main bronchus
20. Spinal cord
1.2.13 Case 13

1. Metabolically active primary tumor in the left upper lobe. Note the posterior necrotic component of the mass in the follow-up study
2. Right main bronchus
3. Left main bronchus
4. Small amount of left pleural effusion
5. Left mastectomy post-op changes
6. Increased activity at nodular lesion in the right perirenal space
7. Thoracic vertebral body osteophyte
8. Left pulmonary hilum
9. Ascending colon
10. Right kidney, inferior pole
11. Aortocaval space
12. Right psoas muscle
13. Left ureter
14. Left perirenal fat
15. Descending colon
16. T11 right costovertebral junction
17. Spinal canal
18. Right diaphragmatic crus
19. Caudate lobe
20. Left hepatic lobe
21. Increased activity at nodular lesion in the left diaphragmatic crus
1.2.14 Case 14

1. Marked increased activity at diffuse left pleural nodular thickening
2. Increased activity at left major fissure involvement
3. Increased activity at the deep left costophrenic angle involvement
4. Sternum
5. Ascending aorta
6. Right main pulmonary artery
7. Right scapula
8. Pulmonary trunk
9. Left main bronchus
10. Right pulmonary hilum
11. Gastroesophageal junction
12. Right hepatic lobe
13. Abdominal aorta
14. Ascending colon
15. Trachea
16. Aortic arch
1.2.15 Case 15

1. Hypermetabolic angiosarcoma, right ventricle component
2. Physiologic uptake in the left ventricle wall
3. Trachea
4. Pericardial effusion
5. Bilateral pleural effusion
6. Left ventricle
7. Esophagus
8. Hypermetabolic angiosarcoma, superior vena cava component
9. Sternum
10. Sphenoid sinus
11. Nasopharynx
12. Oropharynx
1.2.16 Case 16

1. Metabolically active oropharyngeal lymphoma
2. Cerebellum
3. Diffuse FDG uptake in the right ventricular wall
4. Diffuse FDG uptake in the left ventricular wall
5. Right atrium
6. Descending aorta
7. Thoracic vertebral body
8. Spinal cord
9. Right costovertebral junction
10. Vertebral right transverse process
11. Vertebral spinous process
12. Right costal cartilage
13. Right ventricle papillary muscle
14. Left rib, lateral arc
15. Left rib, posterior arc
16. Asymmetric or isolated septal hypertrophy, also known as interventricular septal bulge
1.2.17 Case 17

1. Diffusely increased activity at the aortic root
2. Increased activity at the vocal cords
3. Increased activity at the distal esophagus, probable esophagitis
4. Renal pelvis
5. Right middle ureter
6. Right ventricle
7. Left atrium
8. Left pulmonary vein
9. Spinal canal
10. Right atrium
11. Right pulmonary vein
12. Right costovertebral junction
13. Esophagus
14. Descending aorta
1.2.18 Case 18

1. Brown adipose tissue in typical locations: neck, supraclavicular fossa and paravertebral space
2. Right double collecting system
3. Right first rib
4. Trachea
5. Left clavicle
6. Left humeral head
7. Left glenoid
8. Left scapula
9. Thyroid gland, left lobe
10. Left sternohyoid muscle
11. Left pectoralis major muscle
12. Left subclavian vessels
13. Left trapezius muscle
14. Left paraspinal muscles
15. Right third rib posterior arc
16. Liver
17. Right kidney
18. Stomach
19. Spleen
20. Descending colon
21. Mastoid air cells
22. Nuchal ligament
23. Parotid gland
24. Mandible ramus
25. Pulmonary hilum
1.2.19 Case 19

1. Hypermetabolic right paratracheal lymph nodes
2. Diffusely increased activity at bilateral lungs without discernible CT abnormality
3. Metabolically active splenomegaly
4. Hypermetabolic lymph nodes in prevascular area
5. Trachea
6. Esophagus
7. Thoracic vertebral body
8. Left costovertebral junction
9. Left rib posterior arc
10. Anterior junction line
11. Right main bronchus
12. Right lower lobe
13. Left main bronchus
14. Left lower lobe
15. Left hepatic lobe
16. Right hepatic lobe
17. Stomach
1.2.20 Case 20

1. Ascending aorta
2. Brachiocephalic trunk
3. Left common carotid artery
4. Aortic arch
5. Descending aorta
6. Abdominal aorta
7. Iliac bifurcation
8. Common iliac arteries
9. Main pulmonary artery
10. Right pulmonary hilum
11. Sternum
12. Anterior junction line
13. Left atrium
14. Thoracic vertebral body
15. Spinal canal
16. Right costovertebral joint
17. Right renal pelvis
18. Left kidney
19. Right perirenal space
20. Ascending colon
21. Proximal duodenum
22. Stomach
23. Descending colon
24. Left psoas muscle
25. Left paraspinal muscles (multifidus and erector spinae)
1.2.21 Case 21

1. LCH involvement at the left iliac bone and sacral ala
2. LCH involvement at the right iliac bone
3. Prostatic urethra
4. Testes
5. Right main bronchus
6. Anterior junction line
7. Ascending aorta
8. Left pulmonary hilum
9. Left main bronchus
10. Trachea
11. Right lung apex
12. Horizontal lung fissure
13. Right middle lobe
14. Oblique lung fissure
15. Right lower lobe
16. Left ventricle
17. Left diaphragmatic cupola
18. Sigmoid colon
19. Right iliac wing
20. Right iliac tuberosity
21. Sacral ala
22. Abdominis rectus muscles
23. Right anterior superior iliac spine
24. Right iliopsoas complex
25. Right sacroiliac joint
1.3 Abdomen and Pelvis
1.3.1 Case 1

1. Metabolically active esophageal squamous cell carcinoma
2. Right atrium
3. Left atrium
4. Ascending aorta
5. Descending aorta
6. Aortic knob
7. Left main bronchus
8. Fibrotic changes at the right lung apex
9. Liver dome
10. Gastric fundus
11. Sphenoid sinus
12. Nasopharynx
13. Oropharynx
14. Pharynx
15. Trachea
16. Aortic arch
17. Clivus
18. Right main bronchus
19. Pulmonary artery
1.3.2 Case 2

1. Large metabolically active mass in the wall of the distal esophagus
2. Optic nerves
3. Right nipple
4. Right renal pelvis
5. Left ventricle papillary muscle
6. Right ventricle
7. Left ventricle
8. Esophageal lumen
9. Descending aorta
10. Interventricular septum
11. Superior vena cava
12. Left T9 costovertebral junction
1.3.3 Case 3

1. Metabolically active gastric wall thickening: primary gastric adenocarcinoma
2. Hypermetabolic lymph node metastasis at the gastro-hepatic ligament
3. Left hepatic lobe
4. Right hepatic lobe
5. Inferior vena cava
6. Spleen
7. Gallbladder
8. Hepato-duodenal ligament
9. Gastro-splenic ligament
10. Right retrocrural lymph node metastasis
11. Pancreatic tail
12. Left adrenal gland
1.3.4 Case 4

1. Metabolically active bulky stomach mass
2. Falciform ligament
3. Gallbladder
4. Inferior vena cava
5. Left kidney
6. Gastro-hepatic ligament
7. Hepatic flexure of the colon
8. Spleen
9. Right kidney
10. Hypermetabolic aortocaval lymph nodes
11. Hypermetabolic preaortic lymph nodes
12. Hypermetabolic left paraaortic lymph nodes
13. Pancreatic body
14. Abdominal aorta
1.3.5 Case 5

1. Hypermetabolic concentric mass in the distal ileum
2. Urinary bladder
3. Right femoral head
4. Right iliac wing
5. Sacrum
6. Iliac bifurcation
7. Left acetabulum
8. Left femoral shaft
9. Right gluteus medius muscle
10. Right external iliac vessels
11. Rectum
12. Left piriformis muscle
13. Left gluteus Maximus muscle
14. Ascending colon
15. Right psoas muscle
16. Small bowel loops
17. Left levator ani muscle
18. Left femoral artery
1.3.6 Case 6

1. Metabolically active distal transverse colon adenocarcinoma
2. Metastatic mesenteric lymph node
3. Second portion of duodenum
4. Right adrenal gland
5. Right diaphragmatic crus
6. Spleen
7. Transverse colon
8. Pancreatic body
9. Pancreatic tail
10. Pancreatic head, uncinate process
11. Inferior vena cava
12. Proximal small bowel loops
13. Accessory spleen
1.3.7 Case 7

1. Metabolically active sigmoid colon adenocarcinoma
2. Right acetabulum anterior wall
3. Right femoral head
4. Right acetabulum posterior wall
5. Coccygeal vertebral body
6. Subcutaneous fat, anterior abdominopelvic wall
7. Right iliacus muscle
8. Right gluteus medius muscle
9. Right gluteus maximus muscle
10. Mesenteric fat, normal appearance
11. Small bowel loops
12. Descending colon loops
13. Sacrum
14. Right sacral ala
15. Right sacroiliac joint
16. Left paraspinal muscles
17. Left piriformis muscle
1.3.8 Case 8

1. Metabolically active rectal adenocarcinoma
2. Right obturator internus muscle, posterior aspect
3. Prevesical space
4. Prostate gland with dystrophic calcifications
5. Rectum, thickened
6. Right levator ani muscle, puborectalis
7. Left obturator internus muscle, medial aspect
8. Urinary bladder
9. Levator ani muscles, pubococcygeus
10. Seminal vesicles
11. Perirectal fat
12. Coccyx
13. External iliac vessels
14. Perivesical fat
1.3.9 Case 9

1. Hypermetabolic recurred colon cancer in the umbilical port scar
2. Left rectus abdominis muscle
3. Left transversus abdominis muscle
4. Left internal oblique muscle
5. Left external oblique muscle
6. Left psoas muscle
7. Left quadratus lumborum muscle
8. Left erector spinatus muscle
9. Small bowel mesentery
10. Descending colon
11. Small bowel loops
12. Left lumbar neural foramen
13. Abdominal aorta
14. Inferior vena cava
15. Lumbar vertebral body
16. Right vertebral lamina
17. Spinous process
18. Subcutaneous fat, left abdominal wall

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree