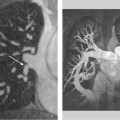
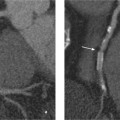
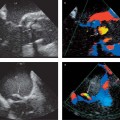
3 Angiography The invasive procedure of angiography is useful in establishing a diagnosis in several cardiac diseases. In addition to selective contrast visualization of the cardiac chambers and coronary arteries, cardiac catheterization enables direct pressure measurements, the measurement of oxygen saturation, and the detection of intracardial shunts. Angiography can further define the full length of the aorta and its branches such as the carotid artery. Digital subtraction angiography (DSA) and electronically enhanced digital imaging techniques are used to improve the quality of the examination. Catheter angiography continues to be the gold standard for vascular imaging, yet the number of conventional angiograms has declined in recent years owing to advances in modern noninvasive procedures such as MR angiography and CT angiography. It can be expected that the number of purely diagnostic catheter angiograms for vascular imaging will continue to decline in the future. Through advances in fast CT scanning in particular, noninvasive imaging of the coronary arteries is also becoming an increasingly important alternative to invasive coronary angiography. Nevertheless, angiography will continue to have an important role in the planning and conduct of interventional procedures. It is to be expected that therapeutic angiography, in contrast to diagnostic angiography, will play an increasingly important role owing to the more frequent detection of abnormalities as a result of the increasing use of noninvasive techniques.1 Except in emergency indications (e. g., acute myocardial infarction), informed patient consent with disclosure of possible risks and complications should be obtained at least 24 hours before angiocardiography. At that time the patient should be asked about allergies, anticoagulant therapy, and possible pregnancy and thyroid complaints. The access site of choice for cardiac catheterization is the femoral artery. This allows retrograde catheterization of the aorta, left ventricle, and all arterial vascular regions of interest. An alternative to the inguinal approach is to introduce the catheter through a brachial or radial artery access. This approach is particularly useful when transfemoral catheterization is not possible (e. g., in patients with severe peripheral arterial occlusive disease [PAOD]). The main advantage of the approach described is that it allows the patient to ambulate immediately after the procedure. Right heart catheterization can be accomplished through a cubital, jugular, or the femoral vein. This permits pressure and oxygen-saturation measurements in the pulmonary circulation, in the right ventricular inflow and outflow tracts, and at various levels in the right atrium. An atrial septal defect or anomalous pulmonary vein terminations can also be catheterized by this approach. Cardiac output can be determined by the thermodilution method or measured indirectly by the Fick principle. Access through the internal jugular vein may be advantageous for right ventricular biopsy. Transseptal cardiac catheterization is generally accomplished through the right femoral vein. A Brockenbrough needle is passed through the interatrial septum, allowing pressure and oxygen-saturation measurements to be taken in the pulmonary veins, left atrium, and left ventricle. This approach is used in particular for diagnostic confirmation, but also for interventional procedures such as balloon angioplasty for mitral valve stenosis. The pressure gradient between the left atrium and left ventricle can be measured simultaneously with a second catheter introduced through the femoral artery and passed in retrograde fashion into the left ventricle. As a rule, angiocardiography is performed under local anesthesia. General anesthesia is required only in rare cases. The femoral artery is usually anesthetized with 10 mL of a local anesthetic solution (e. g., 1% lidocaine). Even smaller amounts may suffice at alternative sites, such as the radial or brachial artery. After a skin wheal has been raised to the left and right of the proposed insertion site, anesthesia of the deep soft-tissue layers is recommended for femoral artery catheterization. An extra-long needle (e. g., a spinal needle) should be used in obese patients. A direct percutaneous approach to the access vessel is made with a suitable beveled needle, which may be open or fitted with an inner stylet. Retrograde puncture of the femoral artery is generally accomplished in slim patients with superficial vessels, but may be difficult in obese patients, or those with severe PAOD. The femoral artery is punctured in the lacuna vasorum in the right or left groin area lateral to the vein, above the median portion of the femoral head. The inguinal ligament is an unsuitable landmark because the targeted common femoral artery runs above it in ~80% of cases. Puncturing the femoral artery above the inguinal ligament may cause initially unnoticed (retroperitoneal) bleeding that is extremely difficult to control by manual compression. The angle of needle insertion depends on the thickness of the soft-tissue layer to be penetrated. A very flat needle angle is generally used in slim patients, while a more vertical angle (up to 90°) is used in obese ones. The needle tip should ideally enter the artery at the first attempt. Multiple puncture attempts increase the risk of complications such as hematomas and arteriovenous fistulas. Care should be taken to puncture only the anterior vessel wall. Puncture of the posterior vessel wall is unfavorable for later manual compression and may lead to retroperitoneal hematoma in a transfemoral approach. A strong, pulsating jet of blood confirms correct intravascular needle placement. Following successful puncture, the access site is secured by inserting a guidewire (usually with a 0.035-inch diameter) through the needle and into the vessel. The guidewire may initially be advanced without fluoroscopic guidance, taking care that it is advanced gently and without resistance. A Judkins J-wire is most commonly used. In severe PAOD—with or without stenosis—the inserted wire may become snared in atherosclerotic plaque. This may require using a special wire with hydrophilic coating, or a straight or slightly curved tip. After removal of the puncture needle, a sheath is inserted into the vessel using Seldinger technique to establish access for angiographic catheterization. The use of a sheath permits various catheters to be introduced as desired without causing additional vascular trauma, and with practically no blood loss. A sheath ~10 cm long is adequate for most diagnostic catheterizations. Longer sheaths can be used if there is significant elongation or kinking of the access vessel (usually the femoral/iliac artery). With technical advances in miniaturization, it has become possible to use sheaths of considerably smaller diameters. Today, 4 Fr and 5 Fr sheaths are most commonly used in diagnostic cardiac catheterizations. Larger sheaths up to 12 Fr or 14 Fr are used for therapeutic angiography (e. g., in angioplasty for coarctation of the aorta). A variety of disposable plastic catheters with various shapes and curvatures are available for accessing the targeted vascular region. A pigtail catheter is essential for imaging the cardiac chambers as well as for aortography. A calibrated pigtail catheter with radiopaque centimeter markings is suitable for aortography to facilitate later quantitative analysis of the angiograms. The catheter is threaded into the vessel over the guidewire. This technique poses fewer risks than advancing the catheter without a wire, which carries a substantial risk of perforation. When the catheter has reached the vessel selected for angiography, the guidewire is withdrawn and the catheter is flushed with heparinized saline solution. Contrast medium can be safely injected only when arterial blood is flowing freely from the catheter. Furthermore, there should be no significant damping of the arterial pressure trace. Before angiography is initiated, correct catheter placement should be confirmed by a test injection of several milliliters of contrast medium. After angiography is completed, the arterial sheath is removed and the insertion site is manually compressed for ~15 minutes until complete hemostasis is achieved. Additionally, a compression dressing is usually applied and left in place for 12–24 hours, depending on the size of the arterial sheath. The insertion site may also be closed with a “closure device,” an increasingly popular option which permits early mobilization even of anticoagulated patients and lowers the risk of post-procedure vascular complications.2 The systolic pressures in the left and right ventricles rise in response to a resistance load in the dependent circulation (e. g., aortic stenosis, coarctation of the aorta, pulmonary stenosis, hypertension). Impaired ventricular function is associated with an increase in end-diastolic pressures. Stenosing valvular lesions and stenoses in larger vessels can be quantified by the simultaneous measurement of pre- and poststenotic pressure differences (e. g., in aortic stenosis). The pulmonary capillary wedge pressure is measured with a balloon catheter carried by the bloodstream (antegrade) into the pulmonary circulation. When the balloon catheter has reached the “wedge” position, the catheter tip measures the pressure in the venous circulatory system distal to the insufflated balloon (the pulmonary veins), which corresponds roughly to the mean pressure in the left atrium. In the absence of mitral valve disease, this pressure equals the left ventricular end-diastolic blood pressure, thereby making it possible to monitor the left ventricular filling pressure. By measuring oxygen saturation at various sites in the pulmonary circulation, we can detect and locate shunt connections between the right and left heart and between the great vessels. We can further quantify the shunt flow. In patients with an atrial septal defect or anomalous pulmonary venous return, the oxygen content of the blood in the right atrium is higher than in the vena cava. In the presence of an isolated ventricular septal defect, the oxygen content in the right ventricle is higher than in the right atrium. With a patent ductus arteriosus, the oxygen content in the pulmonary artery is higher than in the right heart. Cardiac output is measured by the thermodilution method or according to the Fick principle. The thermodilution method uses a pulmonary artery catheter that has an extra port with a separate lumen (central venous lumen) ~30 cm proximal to the catheter tip, and a thermistor probe mounted to the tip. A bolus of cold saline solution is injected, inducing a continuous temperature change that is converted to a change in voltage. When the temperature difference, injection volume, and integral of the voltage change are known, the cardiac output can be calculated using the Stewart–Hamilton formula: CO (L/min) = (1.08) × CT (60) × VI × (TB – TI)/(1.22 × TB(t) dt) The thermodilution method is used today in fully computerized bedside instruments and in all intensive care units. The Fick principle for measuring cardiac output uses oxygen as an indicator. The uptake or release of an indicator by an organ equals the product of blood flow times and the arteriovenous concentration difference. When oxygen uptake is known (from spirometry), the cardiac output can be calculated with the following formula by measuring the arteriovenous oxygen difference: CO = O2 uptake (mL O2/min)/arteriovenous O2 difference (mL O2/L blood) Because the pulmonary veins are accessible only by the transseptal route and the O2 concentration measured in the aorta equals the pulmonary venous oxygen concentration (in the absence of shunt flow), the arterial O2 saturation is used to calculate cardiac output. The following congenital heart lesions can be directly detected by catheterization combined with oxygen saturation measurements and angiography: • Atrial septal defect • Anomalous pulmonary venous terminations on the right atrium • High and low ventricular septal defects • Patent ductus arteriosus In selective angiocardiography, contrast medium is injected directly into a cardiac chamber or central vessel through an angiographic catheter (usually a pigtail catheter) so that the passage of contrast medium can be tracked through all or part of the heart and great vessels. The heart is imaged in two planes (biplane RAO/LAO projections or biplane posterior/left lateral views). Digital images are acquired at a high frame rate (generally 25/s) to compensate for the rapid motion of the heart. Selective left ventricular angiography is usually done as a routine component of coronary angiography and is useful in the assessment of left ventricular systolic function (Fig. 3.1). Left ventricular angiography should precede coronary angiography, because the contrast injection used for coronary angiography may exert a negative inotropic effect and could thus mimic impairment of ventricular function. In addition to the qualitative assessment of regional wall motion abnormalities, this study permits the quantitative computer-assisted determination of end-systolic volume, end-diastolic volume, and stroke volume. These quantities can be used to determine the ejection fraction as a parameter of global systolic function. Left ventricular angiography has further importance in the assessment of mitral regurgitation, which may, however, be an artifact caused also by extrasystoles or the indwelling catheter. Indications for selective left ventricular angiography: • Assessment of regional and global left ventricular function • Left ventricular aneurysm formation after myocardial infarction • Cardiomyopathies • Aortic valve disease • Mitral valve disease
Cardiac Catheterization Technique for Pressure and Oxygen Measurements and for Angiocardiography
 Essential Point
Essential Point
 Essential Point
Essential Point
Cardiac Catheterization Measurements
Pressure Measurements
Oxygen Saturation Measurements
Cardiac Output and Derivative Parameters
Detection of Shunt Lesions
Selective Angiocardiography
Left Ventricular Angiography
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree