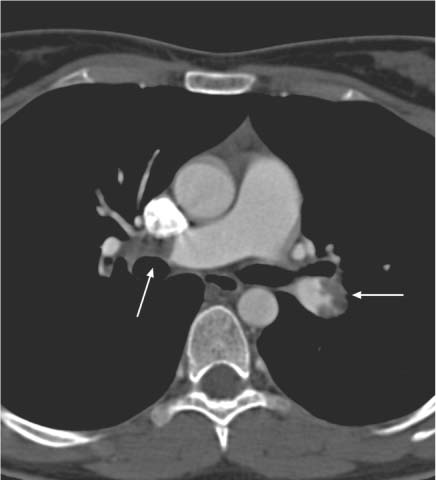
12 Diseases of the Great Pulmonary Vessels The right ventricular outflow tract opens into the pulmonary trunk, which then divides into the right and left main pulmonary arteries. The pulmonary arteries (PA) undergo further branching out to the level of the subpleural space. After the blood has passed through the alveolar capillary bed, the pulmonary veins (PV) return the oxygenated blood to the left atrium. Various diseases may cause an interruption of blood flow on both the arterial and venous sides of the circulation. Besides intravascular causes, primary extravascular pathologies may also lead to a reduction of blood flow. Over time a decrease in the cross-sectional area of the PA or PV leads to an increased resistance in the pulmonary vascular bed, causing the development of pulmonary hypertension (PH). As a result, even primary vascular changes are frequently associated with cardiac changes (e. g., right ventricular hypertrophy, paradoxical septal motion; see Pulmonary Arterial Hypertension, p. 25). This right heart strain usually leads to delayed contrast wash-in, making it necessary to administer a test bolus or use individual bolus timing to achieve a high image quality e. g., in contrast-enhanced CT or MR angiography. Echocardiography is excellent for detection of the increase in pressure in the pulmonary arterial system. It can also be used for the quantification of pulmonary arterial systolic pressure. The advantage of CT and MRI, on the other hand, is their ability to image all the arterial and venous components of the pulmonary vascular bed.1 These modalities can provide an accurate diagnosis of intravascular changes to the subsegmental level as well as extravascular abnormalities. Pulmonary embolism is defined as the embolic occlusion of central or peripheral (segmental to subsegmental) pulmonary arteries. The principal cause is venous thrombosis. Almost all pulmonary emboli originate from thrombi in the iliac veins or deep lower extremity veins, and so both diseases should be viewed as a common entity. Lower extremity venous thrombosis has an annual incidence of about three cases per 1000 population, while pulmonary embolism has a reported incidence of 0.5 cases per 1000 population in Western industrialized countries. It is reasonable to assume, however, that a substantially higher number of clinically silent cases remain undiagnosed.2 From 8% to 12% of symptomatic patients die from the sequelae of acute pulmonary thromboembolism. Numerous risk factors for venous thromboembolism have been identified. They include specific coagulopathies such as factor V Leiden (APC resistance) and hyperhomocysteinemia as well as general risk factors such as age, obesity, and sedentary lifestyle. The clinical complaints associated with acute pulmonary embolism are nonspecific and range from an asymptomatic course to a fulminant, acutely life-threatening condition, depending on severity and location. Frequent clinical symptoms may include: • Dyspnea • Tachypnea • Tachycardia • Chest pain • Syncope Given the nonspecific clinical presentation, imaging studies are particularly important in differentiating pulmonary embolism from other cardiopulmonary diseases such as myocardial infarction, pneumothorax, pneumonia, heart failure, and aortic dissection. An efficient diagnostic work-up must include an analysis of the overall clinical presentation and individual factors (e. g., outpatient or in-patient, age, underlying diseases, etc.). Several studies have shown that an accurate assessment of the clinical likelihood of an acute pulmonary embolism (pretest clinical probability) can make the diagnostic evaluation more cost-effective and easier for the patient to tolerate (e. g., less need for invasive pulmonary angiography).3 An effective diagnostic algorithm for acute pulmonary embolism also depends on factors such as the local availability of imaging procedures (pulmonary angiography, CT, scintigraphy) and operator expertise. Embolic occlusion of the pulmonary arteries has immediate effects on the cardiovascular and respiratory system. The effects include a sudden rise in pulmonary artery pressure with an overload on the right heart. This may progress to an acute decompensation of the right heart with myocardial ischemia, depending on the severity of the embolism and the presence of preexisting disease. The hemodynamic stress may also cause dilatation of the pulmonary artery and right heart, paradoxical systolic motion of the septum toward the left ventricle, possible tricuspid regurgitation, and absence of inspiratory collapse of the inferior vena cava. A decline in left ventricular stroke volume and fall in blood pressure have also been reported.2 Additionally, the ventilation–perfusion imbalance in the lung and the development of intrapulmonary shunts lead to hypoxemia and respiratory insufficiency. Pulmonary embolism is classified into four grades of clinical severity: Grade I involves peripheral pulmonary arteries and is clinically silent in 80% of cases. Grade II is characterized by vascular obliteration at the level of the segmental arteries. The clinical complaints (dyspnea of acute onset, tachypnea, chest pain) overlap with grade III, in which a main branch of the pulmonary artery (left or right PA) or multiple lobar arteries are occluded. The difference between the two grades lies in the blood pressure, which is normal to low in grade II and low in grade III. The mean PA pressure is usually normal in grade II and is normal or slightly elevated (25–30 mmHg) in grade III. Grade IV is an occlusion of the pulmonary trunk or of a main pulmonary branch and multiple lobar arteries leading to cardiac arrest. Echocardiography is a simple, noninvasive, fast, and cost-effective imaging study whose main role in pulmonary embolism is the assessment of right cardiac function. It is the procedure of choice for patients with right heart failure whose life depends on immediate thrombolytic therapy or emergency surgery. Echocardiography is also useful for detecting thrombi in the pulmonary trunk. In principle, direct visualization of the embolus can be accomplished only in the central pulmonary arteries (trunk and main arteries), depending on the mode of use (transthoracic or transesophageal). In most cases the pulmonary arteries cannot be adequately evaluated because of the limited acoustic window. Signs of pulmonary embolism can be found when at least 30% of the pulmonary vascular tree has become occluded. Indirect signs result from the acute pressure load on the right ventricle and the corresponding hemodynamic signs (see above). Multislice CT scanners are becoming increasingly available and can provide an excellent assessment of central and subsegmental pulmonary arteries. Besides confirming or excluding pulmonary embolism, CT angiography (CTA) is also useful in estimating the age of the thrombotic material. Fresh thromboembolic material typically appears on CTA as an intravascular filling defect that partially obstructs the vessel lumen (Fig. 12.1, Table 12.1). The thrombus may occupy the center of the lumen or may be partially adherent to the vessel wall. Complete luminal obstruction appears as an abrupt cutoff, often with no direct evidence of a thrombus. As the thrombotic material ages and anticoagulant or thrombolytic therapy is instituted, the thrombus becomes layered along the vessel wall. Its shape changes over time from convex to concave. Thrombus age in a complete vascular occlusion is much more difficult to assess. Dilatation of the occluded vessel beyond its normal diameter is suggestive of a fresh pulmonary embolism (or intraluminal neoplasm), whereas a reduction in vascular diameter is typical of a chronic embolism. Thromboembolic wall thickening, irregular wall contours, and intraluminal webs and bands are consistent with a diagnosis of chronic recurrent pulmonary embolism. Calcifications of the thromboembolic material occur in ~10% of cases.4 Changes in the lung parenchyma and cardiac morphology5 provide indirect evidence of pulmonary embolism. These changes include the presence of round or oval pleural-based densities in the lung parenchyma, which represent pulmonary infarctions. Associated hemodynamic changes can be quantified by ECG-triggered multislice CT. The thrombus burden itself does not indicate the severity of the hemodynamic stress.6 Taken together, the following four criteria are useful in assessing the severity of pulmonary embolism by CTA: • Vascular obstruction index • Minimum diameter of the left ventricle • Ratio of the smallest diameters of the right and left ventricles • Diameter of the central pulmonary arteries7 Pleural effusion, pericardial effusion, and/or ascites may also be present in various combinations. Fig. 12.1 CT angiogram in acute pulmonary embolism demonstrates mural thrombi in the left lower lobe artery (arrow) and right central pulmonary artery (arrow). The basis for an accurate diagnosis, of course, is a complete examination that provides images of acceptable quality. In particular, the pulmonary arteries should be well opacified and completely visualized. Respiratory artifacts are a significant obstacle to image interpretation, but in most cases the central portions of the pulmonary arteries can be evaluated. Approximately 10% of all examinations are nondiagnostic as a result of technical errors. Multiplanar reformatted (MPR) images are often helpful in making an accurate assessment. In some cases the peribronchovascular tissue may enhance after IV contrast administration, making it more difficult to distinguish between an intraluminal and extraluminal filling defect. Pulmonary veins that are not completely opacified can mimic an intraluminal arterial filling defect. The pulmonary artery can be identified in these cases on the basis of its parallel relationship to the bronchi. MPR images should also be used for the assessment of horizontal oriented pulmonary arteries (lingula, middle lobe, segments 3 and 6). Otherwise, partial volume effects in these vessels could mimic intraluminal filling defects. This problem is less significant in modern multislice CT imagers with an appropriate thin collimation. The presence of pulmonary embolism can be accurately confirmed or excluded in 91% of examinations when both direct and indirect signs are interpreted. Initial data from studies performed with a 16-slice CT scanner document the increased potential of CT. With a slice thickness of 0.75 mm, the subsegmental pulmonary arteries could be directly visualized in 92% of the patients examined. CTA is contraindicated in ~12% of cases. One reason is risk of acute renal failure in response to intravenous contrast medium, for example, in diabetics who take metformin-containing drugs.8 Also, iodinated contrast media should not be used in patients with hyperthyroidism owing to the risk of symptomatic hyperthyroidism or thyrotoxic crisis. Special postprocessing algorithms are available in CT that can map lung perfusion by providing a 3D representation of parenchymal lung enhancement. This requires the acquisition of two complete datasets, the first without contrast medium and the second after IV contrast administration. To date this has been done only for illustrative purposes and is not yet part of the routine postprocessing of CT datasets. In the past, MRI has had only a minor role in the investigation of acute pulmonary embolism. This has been due to the poorer spatial resolution of MRI compared with multislice CT, the longer acquisition times, and problems related to scanner design and the magnetic field making it difficult to handle critically ill patients. But with constant improvements in scanner technology and pulse sequences, the spatial resolution and image quality of MR angiography (MRA) have improved significantly in recent years, making MRA a possible alternative to CTA in the investigation of acute pulmonary embolism especially in cases where CTA is contraindicated.9
Pulmonary Arterial Disorders
Acute Pulmonary Embolism
Risk Factors
Clinical Features
Diagnosis
Pathophysiology
Clinical Classification
Echocardiography
CT Angiography
Limitations and Difficulties of Interpretation
Contraindications
Perfusion CT
Magnetic Resonance Imaging
Acute PE | Central intraluminal filling defects |
| Parenchyma: rounded pleural-based densities, pleural effusion |
Subacute PE | Convex mural filling defects |
| Parenchyma: oval pleural-based densities, pleural effusion |
Chronic PE | Concave mural filling defects, intraluminal “rope ladder” Irregular wall thickening, abnormal vascular tapering, variation in size of segmental vessels |
| Parenchyma: translobular lines, mosaic perfusion, pleural effusion |
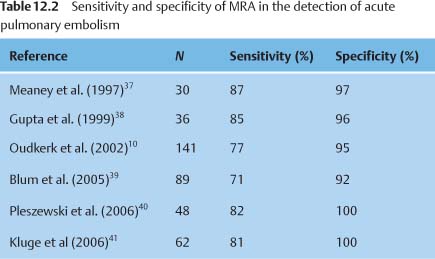
Several studies comparing contrast-enhanced (CE-) MRA with conventional pulmonary angiography have documented the high sensitivity and specificity of the technique (Table 12.2). A recent study found that the accuracy of MRA was still limited in the diagnosis of isolated subsegmental emboli (40% sensitivity).10 Nevertheless, MRA is indicated in selected patient groups owing to its capacity for functional measurements and the absence of radiation exposure and iodinated contrast media. These indications include the following:
• Cases in which radiographic contrast material are contraindicated
• The follow-up of acute pulmonary embolism in patients on anticoagulant therapy
• Preoperative imaging in patients with chronic thromboembolic pulmonary hypertension11
Image Display and Interpretation
As in CTA, the diagnosis of CE-MRA is based on the source images. Viewing images digitally on a monitor is advantageous for reading the images and reporting findings. MPR and MIP reconstructions can also be obtained as an adjunct to source image analysis.
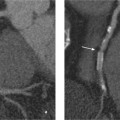
Fresh thromboembolic material in MRA typically appears as a filling defect that occupies the center of the lumen or is partially adherent to the vessel wall (Fig. 12.2). Depending on its extent, the thrombus may completely occlude the vessel and produce a peripheral cutoff sign. Older thrombi appear as irregular mural deposits that contrast with the enhancing vessel lumen. Changes associated with pulmonary embolism, such as pleural effusion and atelectasis, can also be visualized.
Limitations and Difficulties of Interpretation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree