9 Cardiomyopathies and Myocarditis Cardiomyopathies are defined as primary myocardial diseases that are diagnosed after the exclusion of valvular, pericardial, and coronary causes. Typical clinical and hemodynamic features must additionally be present before a cardiomyopathy is diagnosed.1 Although coronary heart disease is still the leading cause of heart failure, the reported incidence of cardiomyopathies has been rising owing to improved diagnostic methods and heightened awareness.2 The joint classification of the ISFC (International Society and Federation of Cardiology) and WHO (World Health Organization) defines cardiomyopathies as diseases of the myocardium that are associated with cardiac dysfunction.3 Primary cardiomyopathies are classified into the following five groups (Table 9.1): • Dilated cardiomyopathy (DCM). Dilated cardiomyopathy is the most common form. It is characterized by dilatation and contraction abnormalities of the left ventricle and presents clinically as heart failure. • Hypertrophic cardiomyopathy (HCM). Systolic function in HCM is initially intact. The disease is characterized by left ventricular hypertrophy, which is frequently asymmetric and mainly involves the interventricular septum. A special form of HCM is hypertrophic obstructive cardiomyopathy (HOCM) with dynamic narrowing of the left ventricular out-flow tract. • Restrictive cardiomyopathy (RCM). Relatively rare in industrialized western countries, RCM is characterized by an abnormality of left ventricular diastolic relaxation. • Arrhythmogenic right ventricular cardiomyopathy (ARVC). This is essentially a dysrhythmic condition that is best demonstrated by cardiac MRI. • Unclassified cardiomyopathies. This category includes fibroelastosis and ventricular noncompaction. Specific or secondary cardiomyopathies result from other cardiac or systemic diseases. Examples are hypertensive, ischemic, valvular, and inflammatory cardiomyopathy. They differ from the primary or idiopathic cardiomyopathies listed above, whose causes are not yet fully understood. Increasingly, however, researchers are describing genetic abnormalities, autoimmune disorders, and chronic forms of myocarditis that contribute to the etiological classification of these diseases (see Table 1.10). Transthoracic echocardiography is the imaging procedure of first choice in cases with satisfactory conditions for ultrasound scanning. This widely available and cost-effective technique provides morphological and functional information on the cardiac chambers and valves that is crucial in narrowing the differential diagnosis. Invasive coronary angiography is still necessary for the definitive exclusion of coronary heart disease in patients with dilated cardiomyopathy. Endomyocardial biopsy is recommended in patients who have rapidly progressive heart failure and/or may require a change in treatment. Owing to the delayed inflow and outflow kinetics of damaged ventricular myocardium and the increased distribution volume that is available for extracellular contrast agents, this tissue shows a further rise of T1-weighted signal intensity following the IV injection of gadolinium chelates. The best pulse sequences for this application are segmented gradient-echo sequences with an inversion-recovery prepulse to null the signal of normal myocardium. In delayed images acquired after contrast administration, viable, healthy myocardium shows low signal intensity whereas damaged myocardium shows high signal intensity. This technique, called delayed-enhancement imaging, can detect myocardial damage due to acute and chronic infarction, inflammatory necrosis, and myocardial fibrosis with a spatial resolution superior to all other imaging techniques.4,5 Dilated cardiomyopathies (DCMs) are characterized by pronounced dilatation of the left ventricle with impaired systolic and diastolic function. In many cases the right ventricle is also enlarged and functionally impaired. The rare cases in which systolic dysfunction is accompanied by only minimal enlargement of the left ventricle are placed in the category of unclassified cardiomyopathies.
Cardiomyopathies
Dilated Cardiomyopathy
Epidemiology, Pathophysiology, and Clinical Features
Main types | • Dilated cardiomyopathy • Hypertrophic cardiomyopathy • Restrictive cardiomyopathy • Arrhythmogenic right ventricular cardiomyopathy • Unclassified cardiomyopathies |
Specific cardiomyopathies | • Ischemic cardiomyopathy • Valvular cardiomyopathy • Hypertensive cardiomyopathy • Inflammatory cardiomyopathy • Metabolic cardiomyopathy • Cardiomyopathy in muscular dystrophy • Cardiomyopathy in neuromuscular disorders • Toxic cardiomyopathy • Cardiomyopathy in pregnancy |
The incidence of DCM is five to eight cases per 100 000 population per year, although this figure does not take into account mild, asymptomatic cases that go unreported. DCM is a common incidental finding in screening examinations.6 Aside from occasional spontaneous recoveries, DCM in symptomatic patients pursues a chronic progressive course with an annual mortality rate of 11–13%.2
Left ventricular dilatation and dysfunction, right ventricular involvement, and maximum oxygen consumption during spiro-ergometric exercise correlate with the prognosis. Most secondary cardiomyopathies (toxic, metabolic, inflammatory, etc.) present functionally as DCM. One example is cardiac hemochromatosis. Hemochromatosis is characterized by an iron overload in parenchymal organs (liver, pancreas, spleen, gonads). The degree of cardiac involvement is highly variable and does not correlate with the involvement of other organs. The cytotoxic effects of iron lead to progressive left ventricular dilatation and dysfunction, which is reversible in response to treatment. Cardiac MRI is particularly useful because the iron overload directly affects magnetic resonance imaging and is easily identified by an early loss of signal intensity in T2-weighted sequences.
The cause remains unclear in over half of patients with DCM. Classified as idiopathic, these cases are attributed to genetic factors, viral myocarditis, and autoimmune mechanisms. For example, genetic mutations with a predominantly autosomal dominant inheritance and very heterogeneous changes in the myocyte cytoskeleton and contractile proteins are found in 25–30% of patients with dilated cardiomyopathy.7
Histological examination reveals pronounced interstitial and perivascular fibrotic changes that occur predominantly in the subendocardium. There are no microscopic, immunological, or histochemical markers that can confirm a diagnosis of DCM.
Imaging
DCM is often noted incidentally on chest radiographs (see p. 22). The imaging procedure of choice for initial diagnosis and follow-up is echocardiography (Fig. 9.1). Transthoracic echocardiography is used to assess the size and function of the ventricles and exclude associated valvular and pericardial diseases.
Typical echocardiographic features of DCM are as follows:
• Spherical dilatation of the left ventricle with a normal wall thickness. Note: The increased diameter of the left ventricle may still be within normal limits by M-mode echocardiography in the early stage of DCM.
• Enlarged left ventricular outflow tract with increased E-point septal separation (EPSS). That is, the distance between the septum and anterior mitral valve leaflet in early diastole is increased on M-mode examination (normal ≤7 mm).
• Loss of “square wave” opening and premature closure of the aortic valve on M-mode echocardiography in advanced stages.
• Left ventricular systolic dysfunction (global reduction of inward systolic motion and wall thickening) with a decreased ejection fraction.
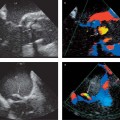
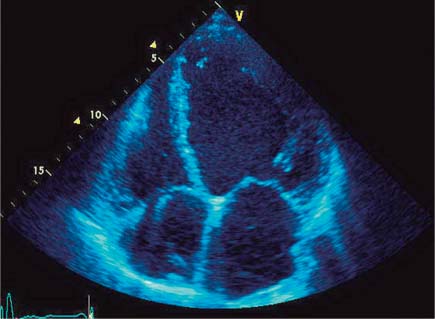
Fig. 9.1 Echocardiography. End-diastolic four-chamber view of a spherically dilated left ventricle in DCM.
• Diastolic dysfunction is another feature of DCM and may range from delayed relaxation to a restrictive filling pattern with a poor prognosis.
• A good parameter for the follow-up of ventricular function is the time ejection index (TEI), or myocardial performance index (MPI), which is determined from the pulsed Doppler waveform of the left ventricular outflow tract and mitral inflow. It is defined as the ratio of the sum of the isovolumetric relaxation and contraction times to the ejection time (normal <0.49).
• Incomplete coaptation of the mitral valve leaflets due to lateralization of the papillary muscles with central mitral valve regurgitation.
• Enlargement of the right ventricle and right atrium with tricuspid regurgitation is an echocardiographic feature of advanced DCM with a poor prognosis.
• Spontaneous echo contrast or left atrial/left ventricular thrombi.
Transthoracic echocardiography is useful in selecting patients with a left bundle branch block and DCM for resynchronization therapy with a biventricular pacemaker system. With its high temporal resolution, this study can supply critical information on the degree of inter- and intraventricular asynchrony. Analysis of myocardial contractions with tissue Doppler is an essential part of this examination.8
The following echocardiographic criteria are predictive of a positive response to biventricular pacemaker stimulation:
• Interventricular asynchrony: delay of 40 ms or more between left and right ventricular ejection, determined from the pulsed Doppler waveforms of the left and right ventricular outflow tracts in relation to the ECG.
• Intraventricular asynchrony: contraction delay of at least 130 ms between the anteroseptal and posterior segments on M-mode echocardiography.
• Subjective impression of a “swinging left ventricle” (blood shifting from one side of the ventricle to the other) on B-mode echocardiography (apical four-chamber view).
The advantages of cardiac MRI over echocardiography are improved delineation of the myocardium and ventricular cavity, especially in the apical and lateral wall regions of the left ventricle; improved visualization of the right ventricle; and good image quality regardless of patient constitution (Fig. 9.2).
With its ability to provide complete and reliable coverage of the left and right ventricles in contiguous short-axis slices, cardiac MRI is the modality of choice for the assessment of regional and global ventricular function. The accurate quantification of end-diastolic and end-systolic ventricular volumes, muscle mass, and ejection fraction on MRI using the Simpson method permits the optimum follow-up of even minor changes9,10 like those described during treatment with growth hormones or ACE inhibitors.11
Ischemic cardiomyopathy is the leading cause of heart failure in elderly patients and the most important condition requiring differentiation from DCM.6 The differentiation of ischemic and nonischemic cardiomyopathies generally relies on invasive coronary angiography. DCM is diagnosed by exclusion based on the absence of coronary stenoses on invasive imaging. The detection of coronary heart disease not only implies a poorer prognosis but also raises other therapeutic options such as interventional or surgical myocardial revascularization, aneurysmectomy, and specific pharmacological therapy with aspirin and statins.
Contrast-enhanced cardiac MRI is also useful in the differentiation of ischemic and nonischemic cardiomyopathies. Thus, areas of delayed enhancement due to an ischemic cause (postinfarction scars) are always subendocardial based on the distribution of myocardial perfusion, whereas delayed enhancement at a subepicardial or intramural site indicates fibrosis due to a nonischemic cause (Fig. 9.3).12
McCrohon et al. examined 90 patients with clinical signs of stable heart failure and left ventricular systolic dysfunction.13 Twenty-seven patients were classified as having ischemic cardiomyopathy by angiography, and 63 were classified as having DCM. Subendocardial areas of delayed enhancement were found in all patients (100%) with coronary heart disease. Three patterns of delayed enhancement were found in the patients classified as DCM by angiography: no enhancement (59%), subendocardial enhancement matching the ischemic pattern (13%), or midventricular enhancement (28%). Thus, MRI goes beyond the capabilities of pure “luminography” in catheter angiography and identifies 13% of patients as having left ventricular dysfunction caused by CHD based on an infarction-type pattern of delayed enhancement. The pathogenic mechanism in these cases may be coronary recanalization after infarction or coronary embolic events. Midventricular delayed enhancement or absence of delayed enhancement can classify patients with heart failure and left ventricular systolic dysfunction as DCM, probably without a need for invasive testing.
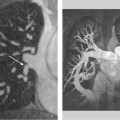
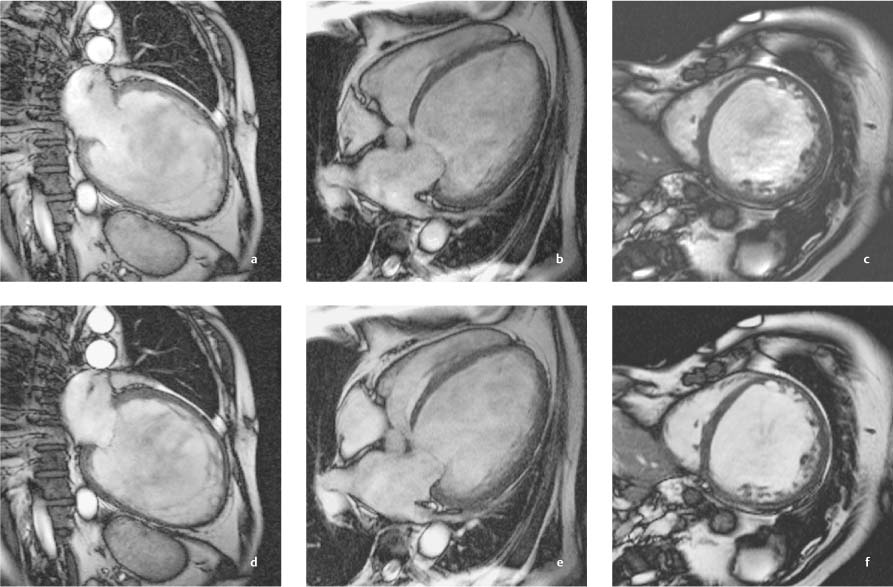
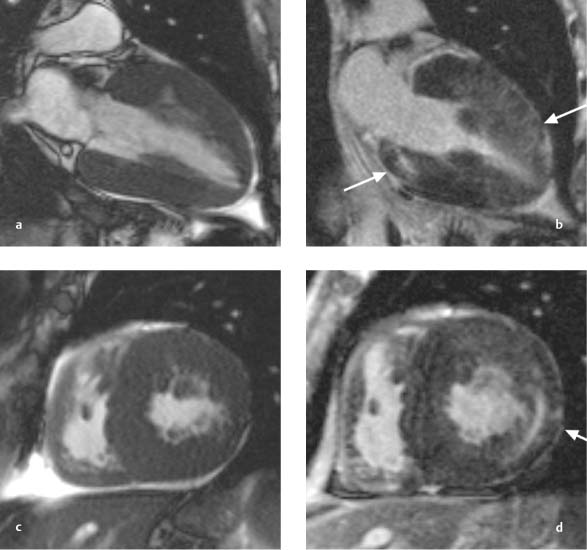
Fig. 9.2a–f Massive left ventricular dilatation in DCM with an end-diastolic volume of 854 mL and a greatly reduced ejection fraction of 6.4%.
a, d Vertical long-axis images in end diastole and end systole.
b, e Horizontal long-axis images in end diastole and end systole.
c, f Sagittal short-axis images in end diastole and end systole.
Apart from that, recent studies suggest that the presence and extent of delayed enhancement predict prognosis in DCM.14
Myocardial tagging with MRI can demonstrate ventricular asynchrony in patients with left bundle branch block. The detection of delayed-enhancing areas in the potential target area of the left ventricular electrode can explain the lack of response to resynchronization therapy in some patients.15
In summary, cardiac MRI can provide the following essential information in the routine clinical investigation of DCM:
• Accurate and reproducible ventricular geometry for optimum follow-up after medical or surgical treatment
• Exclusion of ischemic cardiomyopathy
• Detection and quantification of right ventricular involvement
• Detection of complications such as thrombus or pericardial effusion when echocardiography is unrewarding
• Detection of asynchrony prior to resynchronization therapy in patients with DCM and left bundle branch block (LBBB)
 Essential Point
Essential Point
Typical features of DCM are left ventricular dilatation and dysfunction with a normal myocardial wall thickness. Besides ECG findings of LBBB, it is important to detect inter- and intraventricular dyssynchrony by echocardiography. MRI permits the accurate quantification of ventricular volumes during follow-up. Delayed-enhancement imaging can noninvasively differentiate between CHD and DCM as the cause of ventricular systolic dysfunction.
Hypertrophic Cardiomyopathy
Epidemiology, Pathophysiology, and Clinical Features
HCM is a genetically transmitted myocardial disease that has an autosomal dominant mode of inheritance in at least 50% of cases and a prevalence of 1: 500 in the general population.1
Various mutations of myocyte contractile proteins lead to compensatory hypertrophy, which is characterized histologically by an irregular array of hypertrophic myocytes, diffuse and focal areas of fibrosis, and abnormal intramural coronary arteries with thickened walls and reduced lumina.
HCM may present clinically with angina pectoris, exertional dyspnea, syncope, cardiac arrhythmias, and sudden cardiac death, which often occurs without premonitory signs. HCM is the most common sports-related cause of death in young athletes.16
Hypertrophic cardiomyopathy presents on clinical cardiac imaging with myocardial wall thickening, which is disproportionate in relation to the hemodynamic stress (e. g., aortic stenosis or arterial hypertension) of the left ventricle. Two types of HCM are distinguished—hypertrophic obstructive cardiomyopathy (HOCM) and hypertrophic nonobstructive cardiomyopathy (HNCM)—depending on whether there is dynamic obstruction of the left ventricular outflow tract.
The myocardial hypertrophy in HCM is usually asymmetrical and chiefly affects the interventricular septum and anterolateral free wall of the left ventricle. Approximately 30% of patients have very circumscribed areas of hypertrophy in other left ventricular segments such as the apical region. Occasionally the right ventricle is also involved. In contrast to DCM, systolic left ventricular function is initially in the normal or even high-normal range. The left ventricle in HCM shows characteristic diastolic dysfunction due to the increased stiffness and impaired relaxation of the hypertrophic myocardium. Left ventricular dilatation and systolic dysfunction subsequently develop as the disease progresses. Impairment of left ventricular diastolic filling leads to marked enlargement and hypertrophy of the left atrium and frequently of the right atrium as well.
In ~25% of patients with HCM, a dynamic gradient develops in the left ventricular outflow tract (HOCM). It occurs in midsystole as the anterior mitral valve leaflet moves closer to the thickened interventricular septum (Venturi effect). Typically, mitral regurgitation also occurs owing to the forward systolic motion of the anterior mitral valve leaflet.
Imaging
The imaging modality of first choice is echocardiography. Typical echocardiographic features of HCM are listed below.
• Asymmetrical septal hypertrophy. The ratio of end-diastolic septal thickness to the thickness of the opposing posterior wall is 1.3:1 or more (M-mode).
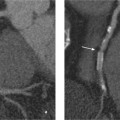
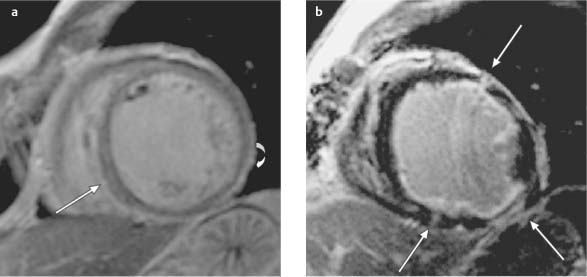
Fig. 9.3a, b Different patterns of delayed enhancement in DCM and CHD.
a Streaks of midmyocardial (straight arrow) and subepicardial delayed enhancement (curved arrow) in a patient with DCM.
b Subendocardial delayed enhancement (arrows) in ischemic cardiomyopathy in a patient with three-vessel coronary disease.
• Banana-shaped left ventricle in the four-chamber view due to septal hypertrophy.
• Parasternal short-axis views show a smooth transition of left ventricular hypertrophy from maximum involvement in the anterior septum to minimally thickened or normal posterior segments.
• Systolic anterior motion (SAM) of the anterior mitral valve leaflet can usually be observed in 2D long-axis images (Fig. 9.4), but M-mode is best for detecting subtle changes and accurately demonstrating the time course, location (chordal, valvular), and degree of septal contact.
• Additional M-mode criteria are midsystolic notching and closure of the aortic valve, and flattened, decreased contraction of the hypertrophic ventricular septum.
• The location of a potential outflow tract obstruction is suggested in color Doppler by the distribution of the hypertrophy and turbulent systolic flow. Pulsed Doppler shows an abnormal increase in systolic flow velocities toward the aortic valve.
• CW Doppler measurements at rest and during stress (ergometry at 75 W, dobutamine) or provocative testing (Valsalva maneuver, nitroglycerin) can accurately quantify the dynamic obstruction of the left ventricular outflow tract. Pressure gradients in excess of 100 mmHg may be documented during stress. Dynamic obstruction of the left ventricular outflow tract (LVOT) typically produces a dagger-shaped signal with a late systolic peak in the CW Doppler waveform over the LVOT.
• Doppler spectra sampled from the outflow tract occasionally indicate presystolic flow, which is attributable to increased atrial contractions arising from left ventricular diastolic dysfunction.
• The systolic anterior motion of the anterior mitral valve leaflet leads to mitral regurgitation, which is detectable by Doppler and color Doppler scanning or by an abnormal position of the papillary muscles.
Rare variants of HCM include the apical form with circumscribed hypertrophy of the apical segments (spadelike appearance in the end-diastolic four-chamber view) and inverted T waves in the left precordial ECG leads. Another variant presents with concentric left ventricular hypertrophy and strictly midventricular obstruction.
The prognosis of HCM depends mainly on the magnitude of the pressure gradient in the left ventricular outflow tract.17 It depends to a lesser degree on septal thickness and atrial size. Because left ventricular diastolic dysfunction is a dominant feature of HCM, it is important to evaluate the left ventricular inflow pattern across the mitral valve and/or in the left ventricle with Doppler echocardiography. Tissue Doppler findings are insensitive to preload and filling pressures. Tissue Doppler measurements of the myocardium close to the mitral annulus can detect diastolic dysfunction of the left ventricle in relatives of HCM patients even before measurable hypertrophy has developed.18
Cardiac MRI is superior to echocardiography for imaging the apical and anterolateral left ventricular segments and is the only modality that can detect atypical phenotypes of HCM with circumscribed hypertrophy of those segments (see Fig. 9.8).19 Just as in DCM, cardiac MRI is ideal for the accurate determination and follow-up of volumetric data such as end-diastolic and end-systolic volumes, ejection fraction, and muscle mass. Thus, an important advantage of MRI is its ability to determine muscle mass and assess the distribution of hypertrophy in the segments of the left and right ventricles (Fig. 9.5). If echocardiography does not provide acceptable image quality, MRI can also demonstrate possible outflow tract obstruction in cine sequences and quantify them with phase-contrast techniques. Additionally, planimetry can be performed in cross-sectional images of the left ventricular outflow tract (Fig. 9.6).
On contrast-enhanced MRI, fibrotic foci in HCM appear as typical intramural streaks or patches of delayed enhancement located in hypertrophic myocardial segments, i. e., predominantly in the septum.20 Delayed enhancement in HCM correlates with clinical risk scores and appears to be an independent risk factor and marker for advanced disease (Fig. 9.7).
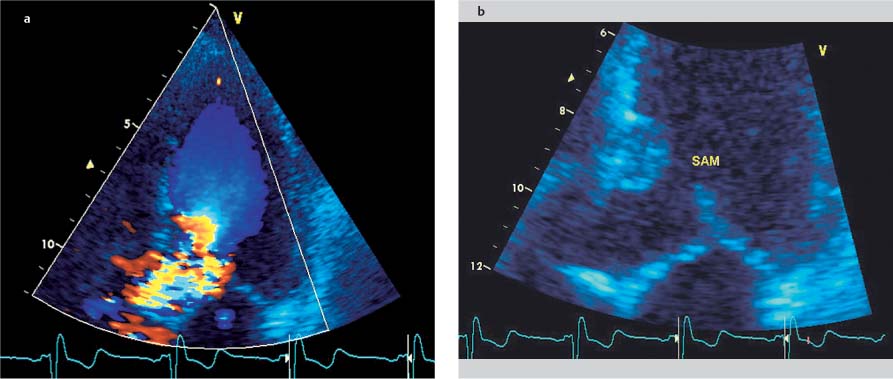
Fig. 9.4a, b Hypertrophic obstructive cardiomyopathy. Turbulent flow acceleration (aliasing) in the left ventricular outflow tract (a) results from dynamic stenosis caused by systolic anterior motion (SAM) of the anterior mitral valve leaflet toward the septum (b).
Fig. 9.5a–d Hypertrophic cardiomyopathy with concentric left and right ventricular hypertrophy. Images after contrast administration (b, d) show both a striate pattern and a patchy, diffuse pattern of delayed enhancement consistent with myocardial fibrosis (arrows).
a, b Vertical long axis.
c, d Sagittal short axis.
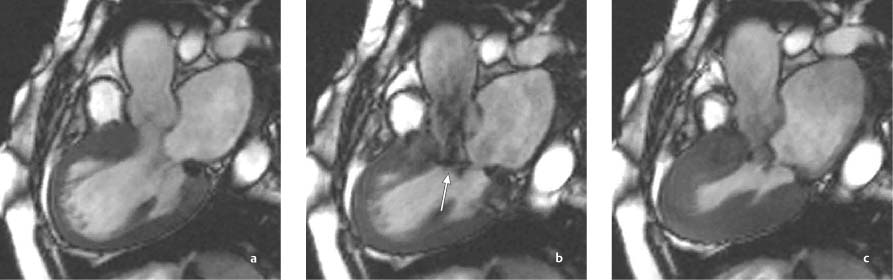
Fig. 9.6a–c Hypertrophic obstructive cardiomyopathy (HOCM). Thickening of the basal septum (a) with systolic obstruction of the left ventricular outflow tract (b, c). A conspicuous jet (arrow) results from turbulent flow acceleration between the septum and anterior mitral valve leaflet during systole (b).
Delayed enhancement is also seen in iatrogenic myocardial necrosis following alcohol embolization or microembolization of a septal arterial branch—a procedure known as transcoronary ablation of septal hypertrophy (TASH).
Gross et al. investigated the cross-sectional area of the left ventricular outflow tract before and after TASH and described the precise anatomical location and extent of necrosis based on delayed-enhancement imaging.21
 Essential Point
Essential Point