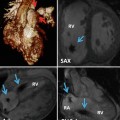
Fig. 24.1
(a) Diagram representing six paired branchial arches (numbers 1–6) and an intersegmental artery (IA). The arches connect paired dorsal and ventral aortae during development. (b) Schematic representation of the Edwards hypothetical double arch. Dual right- and left-sided aortic arches give rise to bilateral common carotid arteries and subclavian arteries as independent structures. The selective involution of arch segments gives rise to the final adult aortic arch and serves as a mechanism to explain several common arch anomalies. LCCA left common carotid artery, LECA left external carotid artery, LICA left internal carotid artery, RCCA right common carotid artery, RECA right external carotid artery, RICA right internal carotid artery, LSA left subclavian artery, RSA right subclavian artery, LDA left ductus arteriosus, RDA right ductus arteriosus, LPA left pulmonary artery, RPA right pulmonary artery, MPA main pulmonary artery
In order to conceptualize how specific perturbations in arch development affect aortic anatomy, it is helpful to rely on the Edwards hypothetical double arch concept (Fig. 24.1b). In this model the developing aorta is represented by dual right- and left-sided arches with ductus arteriosus on each side. Although development of the aortic arch proceeds such that this structure does not exist in its entirety at any one time, it has nonetheless proven helpful in showcasing the effect of interruptions to the developing aorta at specific locations.
Imaging of the Aortic Arch and Associated Structures
Chest Radiography and Echocardiography
Although limited in its ability to provide detailed anatomic information regarding the aorta and its associated vessels, there are several direct and indirect findings on chest radiography (CXR) that may raise the suspicion for an aortic arch anomaly. Evaluation of the position of the aortic knob and para-aortic stripe provides information as to whether the arch is left or right sided, as does the position of the descending aorta. Similarly, in infants and small children, a deviated trachea may serve an indirect sign of compression by an aortic arch opposite the side of deviation. Lateral CXR may suggest a vascular anomaly when there is obscuration of the retrotracheal lucency (an area commonly known as Raider’s triangle) [2]. Along with CXR, echocardiography has been used as a first-line imaging modality to examine pediatric patients for suspected aortic arch anomalies. Echocardiography has the benefit of not utilizing ionizing radiation and the relative ease at which it can be performed at the bedside. However, it is challenging to visualize the entirety of the thoracic aorta, and patient habitus, operator skill, and available sonographic windows may limit the final images. Although at experienced centers echocardiography can be utilized to accurately diagnose vascular rings, local expertise may limit its role to the diagnosis of associated cardiac abnormalities. Rarely, aortic arch anomalies may also be diagnosed in utero through fetal echocardiography [3, 4].
Computed Tomography and Magnetic Resonance Imaging
The advent of ECG gating, multiple detectors and spiral imaging, and radiation dose reduction methods has recently revolutionized computed tomography (CT) imaging. Using these techniques, CT is capable of generating detailed anatomic images in a matter of seconds with isotropic submillimeter resolution that can be used to reconstruct images in any two-dimensional plane. Improvements in advanced post-processing methods utilizing maximum and minimum intensity projections, as well as three-dimensional models with surface and volume rendering methods, allow CT to play an important role in imaging aortic arch pathology. The speed, ease, access, and lack of sedation requirements make CT a reasonable imaging choice in both the adult and pediatric populations. However, CT imaging utilizes potentially carcinogenic ionizing radiation and often requires iodinated contrast agents that on occasion evoke anaphylactoid reactions (about 0.6–0.7/100 adult patients). Iodinated contrast agents are also known nephrotoxic agents, and it should be noted that unlike other imaging modalities such as MRI or echocardiography, CT cannot assess or quantify normal or abnormal blood flow and pattern. Overall, the risk of adverse events from CT scan is likely much lower than the expected benefit for the great majority of patients. The precise technique and scan parameters may depend on the specific CT machine and the type of study. Contrast-enhanced CT angiogram (CTA) is usually performed with thin collimation (0.5–1 mm) and multiplanar images are reconstructed as thin slices of around 1–2 mm. Typically, injection rates of 3–5 ml per second of intravenous iodinated contrast containing are used, through a peripheral intravenous cannula placed in the right upper extremity to minimize streak artifact from dense contrast material in the innominate veins. In most cases, the scan can be triggered by automated bolus tracking by placing the region of interest (ROI) in the aortic lumen, at an attenuation threshold of 150–180 Hounsfield units to achieve reliable aortic enhancement. Cardiac pulsation artifact mainly affects the aortic root in non-gated examinations. Electrocardiogram (ECG)-gated CT may be preferred for the assessment of the aortic root with minimal or no motion-related artifacts. Slowing the heart rate with β-blockers may also be helpful in improving image quality. The penalty of using ECG gating, especially in retrospective gating mode, is an increase in radiation dose.
In addition to morphological assessment, blood flow velocity mapping, directional flow assessment, and pressure gradient calculation are routinely performed in MRI. These examinations can be performed either with or without intravenous gadolinium-containing contrast material. Multiphasic postcontrast evaluation is also possible in MRI. However, drawbacks to MRI examinations include claustrophobia, incompatibility with metallic devices, renal impairment restricting the use of gadolinium (due to the potential for development of nephrogenic systemic fibrosis), and prolonged examination times requiring sedation or, particularly in children, general anesthesia. Relatively limited access, affordability, and expertise may also prohibit the routine use of MRI.
The decision to utilize CT vs. MRI or another imaging modality is made based on individual patient factors as well as institutional preference and expertise.
The Normal Aortic Arch
Typically the aorta originates just left of midline and ascends obliquely and slightly to the right before turning posteriorly and leftward to form the aortic arch. The aorta completes its course by then descending just left of midline adjacent to the anterior thoracic vertebral bodies, exiting the diaphragm through the aortic hiatus. After the coronary arteries, the first major vessel arising from the aortic arch is the brachiocephalic (innominate) artery, which bifurcates quickly to form the right subclavian and right common carotid arteries, followed by the origins of the left common carotid artery and left subclavian artery (Fig. 24.2a). On the right side the primitive ductus arteriosus involutes completely, while on the left it persists to serve as a fetal bypass of the pulmonary vasculature. This typical branching pattern is eventually accomplished through interruption of the Edwards hypothetical double arch distal to the right subclavian artery (Fig. 24.2b).
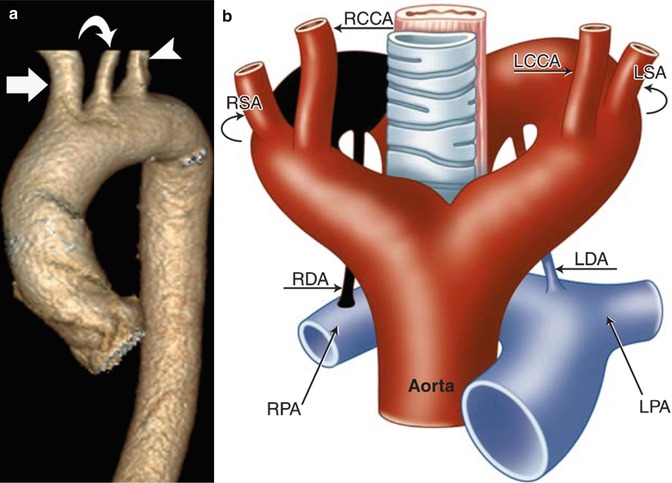

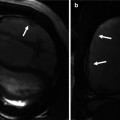
Fig. 24.2
(a) Normal aortic arch morphology. There are three normal great vessels emanating from the arch in the typical fashion: right brachiocephalic artery (arrow), left common carotid artery (curved arrow), and left subclavian artery (arrow head). (b) Schematic representation of the normal aortic arch via the Edwards hypothetical double arch concept. The area shaded in black undergoes involution resulting in the formation of the normal left-sided aortic arch. The right arch segment proximal to the area of involution forms the brachiocephalic trunk, and the persistent left arch segment forms the aortic arch and proximal descending aorta. Typically, the right ductus arteriosus undergoes involution leaving only the left aortic arch with left ductus arteriosus. LCCA left common carotid artery, LSA left subclavian artery, RCCA right common carotid artery, RSA right subclavian artery, LDA left ductus arteriosus, RDA right ductus arteriosus, LPA left pulmonary artery, RPA right pulmonary artery
Normal Variants in Aortic Arch Vessel Branching
Large case studies have demonstrated that this “normal” arch configuration exists in only approximately 65–75 % of the population [5–7]. The most common variant arch vessel branching pattern is the so-called “bovine” aortic arch.
Bovine Aortic Arch
The bovine aortic arch branching configuration occurs when the left common carotid artery is shifted leftward to share a common origin with the brachiocephalic trunk as opposed to arising directly from the aortic arch. Slightly less common is a variant of bovine aortic arch in which the left common carotid artery arises as a branch off the brachiocephalic trunk as opposed to sharing a common ostium [8]. The incidence of bovine aortic arch has been reported in the literature at approximately 15–27 %, with most large studies placing that estimate between 15 and 20 % [5–7]. Both bovine arch variants are generally asymptomatic.
Aberrant Origin of Vertebral Artery
The left vertebral artery will occasionally arise not from the left subclavian artery, but instead will originate directly from the aortic arch. This arch variant is generally asymptomatic, with several studies observing this branching pattern in approximately 1–8 % of the study population [5, 9, 10]. With less frequency, the right vertebral artery may directly arise from the aortic arch. Aberrant origin of the right vertebral artery from the aorta distal to the origin of the left subclavian artery is very rare (Fig. 24.3). It courses behind the esophagus and may have a diverticulum at its origin (vertebral lusoria) [9].
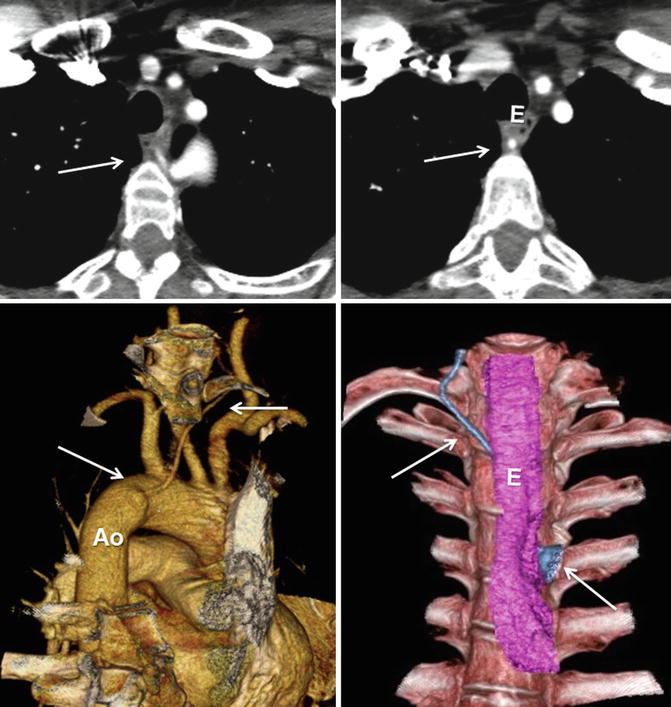

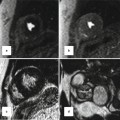
Fig. 24.3
Vertebral arteria lusoria (arrows). Aberrant origin of the left vertebral artery from distal aortic arch (Ao) shown in CT images. Note diverticular outpouching at its origin. The vessel travels obliquely behind the esophagus (E) and may be compressed between the esophagus and vertebra (Courtesy of Farhood Saremi MD, USC)
Anomalous Brachiocephalic Trunk
In this condition the origin of the brachiocephalic artery is displaced just left of midline on the aortic arch. Historically, this condition has been thought to be a cause of respiratory symptoms in infants and young children; however, more recent studies have demonstrated that the brachiocephalic trunk arises left of the trachea in >95 % of newborns and that most infants and young children display some degree of asymptomatic tracheal indentation. Only when tracheal compression exceeds 50 % of the AP diameter is this condition considered potentially pathologic, although some patients may remain asymptomatic even at this degree of compression due to its dynamic nature. While tracheal deformity was believed to be primarily due to the variant brachiocephalic trunk origin, it is now thought to represent a functional anomaly primarily related to immature tracheal cartilage [11]. Most infants and children display few, if any, symptoms related to tracheal compression, although some may develop significant respiratory compromise. When symptoms are clinically significant, the condition is known as innominate artery compression syndrome.
Currently both CT and MRI are able to delineate the aortic arch anatomy as well as quantify the degree of tracheal compression. Unlike bronchoscopy, however, neither can easily visualize tracheal changes throughout the respiratory cycle. In contrast to cross-sectional imaging, bronchoscopy offers no additional relevant information, such as surrounding vascular anatomy or associated cardiac defects.
Symptomatic patients may be treated with surgical suspension of the brachiocephalic trunk to the posterior sternum via a procedure known as retrosternal innominate arteriopexy in order to provide symptomatic relief or reimplantation of the artery in an anatomically correct position on the aortic arch.
Anomalies of the Aortic Arch
Classification of Aortic Arch Anomalies
Aortic arch anomalies may be classified with the Edwards hypothetical double arch, with stratification based upon the point at which the hypothetical arch has been interrupted. Clinically, classification is most likely to proceed on the basis of anatomy and morphology, where the course of the aorta and arch vessels form the basis for classification. In this system, arch anomalies may be characterized as right or left sided, interrupted, double, or cervical, with additional classification based on the pattern of arch vessel branching. Further, anomalies may be characterized as symptomatic or asymptomatic. The majority of aortic arch malformations remain asymptomatic; however, certain anomalies may be symptomatic, commonly due to tracheobronchial or esophageal compression from a vascular ring and rarely due to a cerebral “steal” phenomenon where blood is shunted away from the cerebral vasculature to provide reconstituted flow to an isolated vessel.
Left Aortic Arch
The left aortic arch morphology is described earlier under “The Normal Aortic Arch.”
When classified based on arch vessel branching, there are three configurations of major vessels that are seen with a left-sided aortic arch: normal, aberrant right subclavian artery, and isolated subclavian artery.
Aberrant Right Subclavian Artery
Pathology and Embryology
Aberrant right subclavian artery (ARSA) is the most common form of thoracic arterial anomaly with a prevalence of 0.4–2 % [12]. An ARSA is believed to be the result of complete involution of the right fourth pharyngeal arch and proximal right dorsal aorta, with the right seventh intersegmental artery giving rise to the right subclavian artery from a segment of persistent dorsal aorta. The aortic remnant often persists as a saccular diverticulum known as a Kommerell diverticulum, which in approximately 8 % of patients may become aneurysmal requiring surgical resection [13]. According to the Edwards hypothetical double arch model, the site of interruption occurs between the right common carotid and right subclavian arteries (Fig. 24.4). In order to reach the right upper extremity, the artery takes a right superior oblique course passing behind the esophagus in the majority of cases; however, in rare cases it may pass between the esophagus and trachea or anterior to the trachea [14].
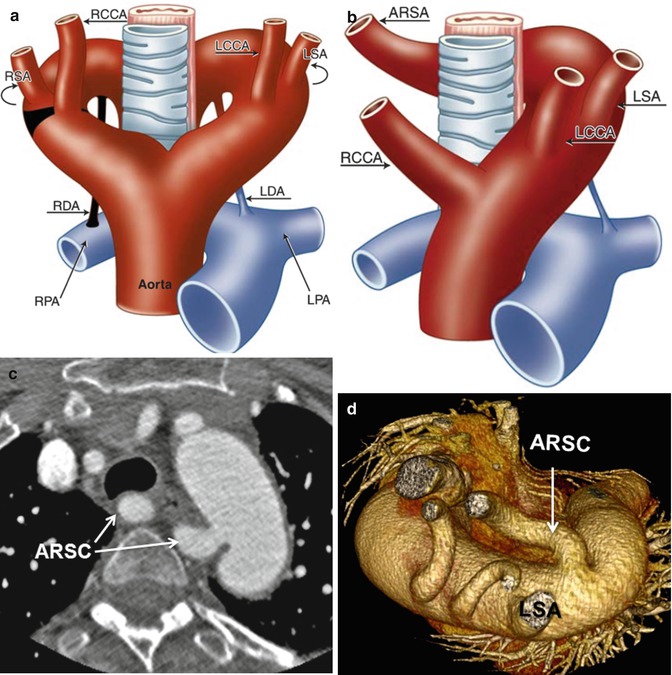

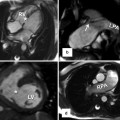
Fig. 24.4
(a, b) Schematic representation of the left aortic arch with aberrant right subclavian artery (ARSA). (a) The black–shaded area represents the break point in the Edwards hypothetical double arch. (c) Axial CT and (d) superior volume-rendered view of the aortic arch demonstrate ARSC coursing behind the esophagus. LCCA left common carotid artery, LSA left subclavian Artery, RCCA right common carotid artery, RSA right subclavian artery, LDA left ductus arteriosus, RDA right ductus arteriosus, LPA left pulmonary artery, RPA right pulmonary artery
Associations
An ARSA occasionally occurs in conjunction with several intracardiac anomalies, the most common of which is tetralogy of Fallot. Among patients with tetralogy of Fallot, the incidence of ARSA is approximately 5–16 %, with rates slightly higher in patients with both tetralogy of Fallot and pulmonary atresia [15–17]. Other associated cardiac defects include left-sided obstructive lesions, atrioventricular canal defects, interrupted aortic arch, and coarctation of the aorta; however, these remain less commonly associated.
Clinical Symptoms
An ARSA is most commonly asymptomatic. Very rarely, infants and young children may develop dysphagia or respiratory symptoms resulting from tracheoesophageal compression. The presence of severe symptoms in a child suggests the presence of an additional lesion forming a complete vascular ring. Causes of this condition include a persistent double aortic arch, common origin of both carotid arteries, or right-sided ductus arteriosus.
Patients in their seventh and eighth decades may develop late dysphagia as the ARSA becomes stiffened and ectatic by atherosclerotic changes and compresses the posterior esophagus. Symptoms of tracheoesophageal compression due to an ARSA have been termed dysphagia lusoria (“prank of nature”) [18].
Imaging
On frontal CXR the findings of an oblique edge or opacity extending to the right from the aortic knob, demonstration of the vessel through the lucency of the tracheal air column, or a mass effect at the medial right clavicular area suggest the presence of ARSA. On lateral CXR, obscuration of the retrotracheal opacity (Raider’s triangle), aortic arch obscuration, and posterior tracheal imprint provide evidence for this condition but remain a poor means of definitive diagnosis [19].
In general most patients will be diagnosed with ARSA incidentally on cross-sectional imaging. Both CT and MRI will demonstrate a vessel emerging from the aortic arch distal to the left subclavian artery that course obliquely, right, and superiorly towards the right axilla (Fig. 24.4). Often slight tracheal or esophageal indentation, and rarely significant tracheoesophageal compression, may be appreciable on imaging.
Treatment
In cases of severe dysphagia, respiratory compromise, or aneurysmal dilation, surgery may be employed to reimplant the anomalous subclavian artery at a more favorable location. Often this is accomplished through a modified right carotid-subclavian artery bypass or reimplantation of the vessel at a more proximal arch position. Commonly, this is now accomplished via a two-stage hybrid procedure involving transcatheter occlusion of the origin of the ARSA followed by open carotid-subclavian bypass [20, 21].
Right Aortic Arch
Right aortic arch occurs when the aortic arch both originates and descends to the right of the vertebral bodies. The incidence of right aortic arch is approximately 0.1 % or less of the population; however, with the advent of MDCT and MRI, some clinicians now believe the true incidence to be higher [22]. Right aortic arch results when the developmental pattern of the fourth branchial arch is reversed. Normally, the left fourth arch forms a portion of the aortic arch, while the right fourth arch forms the right subclavian artery; however, reversal of this pattern results in development of a right aortic arch. Development of right aortic arch may occur in isolation or in association with intracardiac abnormalities, esophageal atresia, or tracheoesophageal fistula. Although frequently asymptomatic, patients may present with a number of symptoms such as dysphagia, dyspnea, hypertension, and heart failure. The molecular etiology of right aortic arch remains poorly understood; however, alterations in embryonic blood flow have been hypothesized to contribute to the condition.
Classification of right aortic arch anomalies has historically been accomplished based on the arrangement of the arch vessels as well as the position of the ligamentum arteriosum [23]. Under this system there are three subgroups of right aortic arch:
1.
Right aortic arch with mirror image branching (type I)
2.
Right aortic arch with aberrant left subclavian artery (ALSA) (type II)
3.
Right aortic arch with isolation of the left subclavian artery (type III)
Within these subgroups, the ligamentum arteriosum is most frequently left sided but may be situated on the right or both sides. If the ligamentum arteriosum is situated on the left opposite the aorta, a complete vascular ring may result in tracheoesophageal compression, a condition known as a “Neuhauser” anomaly.
Right Aortic Arch with Mirror Image Branching (Type I)
Pathology and Embryology
Embryologically, this condition results from disruption of the Edwards hypothetical double arch between the descending aorta and the left subclavian artery (Fig. 24.5). Typically, the aorta both originates and descends on the right side of the thorax, eventually crossing over the anterior thoracic vertebrae inferiorly before exiting through the aortic hiatus.
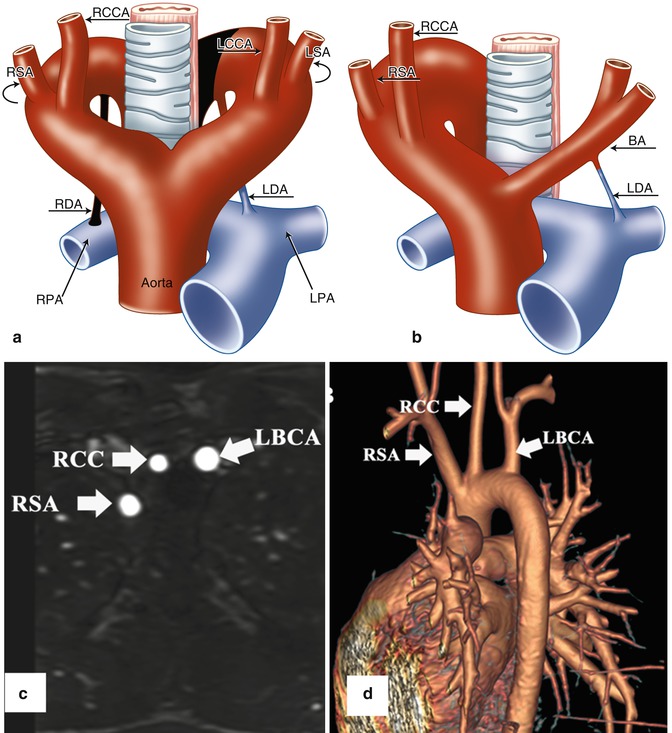

Fig. 24.5
(a, b) Schematic illustration of right aortic arch (RAA) with mirror image branching pattern. (a) The black-shaded area represents the break point in the Edwards hypothetical double arch model. (b) Schematic of the evolved RAA with mirror image branching. (c) Axial reformatted MR angiography and (d) 3D volume-rendered image (posterior oblique view) demonstrate RAA with mirror image branching pattern in an 18-year-old young man with history of tetralogy of Fallot status post repair. LCCA left common carotid artery, LSA left subclavian artery, LBCA left brachiocephalic artery, RCC and RCCA right common carotid artery, RSA right subclavian artery, LDA left ductus arteriosus, RDA right ductus arteriosus, LPA left pulmonary artery, RPA right pulmonary artery, BA brachiocephalic artery
In most cases the ductus arteriosus is left sided, and it is the origin of the ductus that determines whether or not a complete vascular ring is formed [24]. Often a left-sided ductus connects the left pulmonary artery to the left subclavian artery or descending aorta, and there is no complete vascular ring. However, in the rare event that the ductus originates from the posterior aspect of the aorta, a complete vascular ring is formed and may present with tracheoesophageal compression [24]. Occasionally, a patient may present with bilateral ductus arteriosus, in which case a constrictive vascular ring may also be present. Finally, in several instances, a right-sided ductus has been described, in which case there is no vascular ring.
Associations
Based on case reports and case series studies, right aortic arch with mirror image branching is associated with intracardiac malformations in many cases [25, 26]. Most commonly this condition is seen in association with pulmonary atresia with ventricular septal defect (VSD) (46 %), tetralogy of Fallot (32 %), and double-outlet right ventricle with right atrial isomerism (14 %) [27].
Clinical Symptoms
Right aortic arch with mirror image branching is generally asymptomatic, even in cases of left-sided ductus arteriosus and complete vascular ring. In rare cases, infants or young children may present with symptoms of tracheoesophageal compression. Occasionally, patients may become symptomatic late in life due to atherosclerotic hardening of the aortic arch or subsequent compression of mediastinal structures by aneurysmal dilation [28].
Imaging
As most patients with this condition remain asymptomatic, it is often identified incidentally on imaging obtained for unrelated reasons. CXR may demonstrate a right-sided aortic knob with right-sided para-aortic stripe. Occasionally a right-sided aortic arch may mimic a right superior mediastinal mass.
Cross-sectional imaging demonstrates a right-sided aortic arch with a left brachiocephalic trunk emerging as the first arch branch, followed by the right common carotid artery and the right subclavian artery (Fig. 24.5). The brachiocephalic trunk courses anterior to the left pulmonary artery before dividing into the left subclavian and left common carotid arteries. The ductus arteriosus arises from either the posterior aspect of the aortic arch opposite the right subclavian artery or from the origin of the left brachiocephalic artery.
Treatment
In the rare instance that an isolated complete vascular ring is present, treatment may be needed to divide the ligamentum arteriosum. More commonly, treatment of this condition centers on correcting the associated intracardiac defects. Importantly, the surgeon should be made aware of the presence of a right-sided aortic arch prior to intervention requiring a modified Blalock-Taussig shunt as it will be placed on the contralateral side of the arch.
Right Aortic Arch with Aberrant Left Subclavian Artery (Type II)
Pathology and Embryology
Right aortic arch with aberrant left subclavian artery (ALSA) is the most common of the right aortic arch subtypes, and it is the second most common cause of constrictive vascular ring, the first being double aortic arch. In approximately 5–10 % of cases, it may occur in conjunction with intracardiac disease; however, most cases are an isolated phenomenon [26, 29]. Embryologically, interruption of the Edwards hypothetical double arch occurs between the left subclavian and left common carotid arteries (Fig. 24.6). The first major arch vessel to emerge is the left common carotid artery followed by the right common carotid artery, right subclavian artery, and aberrant left subclavian artery. The aberrant left subclavian artery arises at the junction of the right arch and the descending aorta, often from a remnant of the distal left fourth arch.
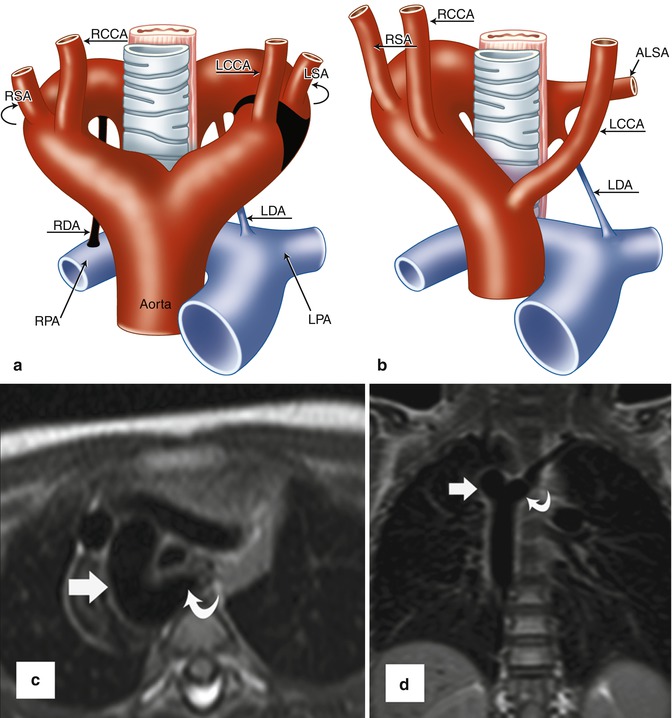

Fig. 24.6
(a, b) Schematic representation of right aortic arch (RAA) with aberrant left subclavian artery (ALSA). (a) The black-shaded area represents the break point in the Edwards hypothetical double arch model. (b) Schematic of the evolved RAA with ALSA. Note the persistent left ductus arteriosus forming a complete vascular ring. (c) Axial and (d) coronal black-blood MR images demonstrate RAA (straight arrows) with ALSA (curved arrows). A large diverticulum of Kommerell is seen posterior to the esophagus. The trachea is mildly narrowed. LCCA left common carotid artery, LSA left subclavian artery, RCCA right common carotid artery, RSA right subclavian artery, LDA left ductus arteriosus, RDA right ductus arteriosus, LPA left pulmonary artery, RPA right pulmonary artery
Most often the ductus arteriosus is left sided and runs from the aortic remnant to the left pulmonary artery, thus forming a complete vascular ring [29]. In many instances the ring remains relatively loose and is asymptomatic; however, in some patients the ring may be sufficiently tight so as to result in tracheoesophageal compression. This is a much more frequent occurrence in children and young adults, with most adults remaining symptom-free. In rare circumstances, the ductus arteriosus may be right sided, in which case there is no vascular ring and patients remain asymptomatic.
Associations
As stated previously, only approximately 5–10 % of cases of right aortic arch with aberrant left subclavian artery are associated with intracardiac disease. Most often this type of arch branching pattern occurs in isolation and may be detected only incidentally on cross-sectional imaging.
Clinical Symptoms
In general, most patients with this condition are asymptomatic. In the setting of a tight vascular ring formed by the left-sided ductus arteriosus, some patients may experience symptoms of tracheoesophageal compression. This is seen more commonly in infants and young adults, with only rare patients presenting in adulthood due to vessel atherosclerosis (most commonly of the carotid arteries) and/or aneurysmal dilation of Kommerell diverticulum [30]. Some of the more severe symptoms in pediatric patients may include poor feeding tolerance, recurrent aspiration, and failure to thrive.
Imaging
Diagnosis of this condition is best accomplished with cross-sectional imaging via MDCT or MRI. On CXR, a right-sided aortic knob and para-aortic stripe with widening of the right superior mediastinum provides evidence for right aortic arch. When present, an aortic diverticulum (Kommerell diverticulum) may present as a soft-tissue density to the left of the spine and may be mistaken for other mediastinal lesions such as tumors [23]. Occasionally on lateral CXR an area of increased opacity in the retrotracheal space may be present due to the aberrant left subclavian artery [22].
As the last of the major arch vessels to emerge from the aortic arch, the aberrant left subclavian artery is seen originating at the juncture of the aortic arch and descending aorta, possibly from a Kommerell diverticulum. After emerging from the aorta, the vessel courses obliquely upward behind the trachea and esophagus, often visibly indenting the esophagus, before assuming its normal trajectory to supply blood to the left upper extremity (Fig. 24.6). Kommerell diverticulum may contribute to esophageal compression and may make it difficult to distinguish between right aortic arch with aberrant left subclavian and double aortic arch with atresia of the left arch [31]. Importantly, double aortic arch is a more common cause of vascular ring, and any uncertainty about the diagnosis should be communicated preoperatively to the surgeon for appropriate surgical planning.
When performing imaging for this condition, it is important to remember that the ligamentum arteriosum may be poorly visible or not at all visible on cross-sectional imaging [32]. Indentation of the left lateral trachea and/or esophagus in the setting of right aortic arch with aberrant left subclavian artery may suggest the presence of a constrictive ligamentum arteriosum; however, it is frequently difficult to identify this structure with any certainty prior to direct visualization in the operating room.
Treatment
Patients with symptoms of tracheoesophageal compression due to vascular ring are candidates for surgical transection of the ligamentum arteriosum. The Kommerell diverticulum is tacked posteriorly to the prevertebral fascia to prevent impingement on the esophagus. If the diverticulum appears enlarged or frankly aneurysmal, it can be resected. This is generally accomplished through a limited left-sided thoracotomy or minimally invasive approach.
Asymptomatic patients with an aneurysmal Kommerell diverticulum are candidates for surgical resection of the diverticulum with reimplantation of the aberrant left subclavian artery to the aorta as the risk of rupture is relatively high. One study of 32 patients reported a rupture rate of 53 % among those with right aortic arch and aberrant left subclavian artery; however, identification of asymptomatic patients with this condition remains challenging and the true denominator is unknown [33]. However, if identified, surgical resection of the aneurysm is recommended when the maximum diameter reaches 3–5 cm in diameter [33, 34]. All symptomatic patients are candidates for surgical resection. Surgical treatment options include both traditional open approaches and a hybrid approach including both an open component and endovascular component. Often, treatment involves left common carotid to left subclavian bypass with transcatheter occlusion of the origin of the ALSA. An associated Kommerell diverticulum may be treated via open aneurysmorrhaphy, graft interposition, or endovascular stent graft placement in candidates with favorable anatomy [30].
Postoperative imaging may demonstrate occlusion of the ALSA at its origin via an occlusion plug with reconstitution of flow via carotid-subclavian bypass. Alternatively, in patients who underwent an open repair, there may be evidence of graft interposition at the site of the resected Kommerell diverticulum with reimplantation of the ALSA directly to the aortic arch or proximal left common carotid artery [30].
Right Aortic Arch with Isolated Left Subclavian Artery (Type III)
Pathology and Embryology
Isolation of a subclavian artery is a rare malformation, with reports in the literature generally being limited to case studies. This condition results from disruption of the Edwards hypothetical double arch both proximal and distal to the origin of the affected artery, with the isolated artery originating from the ipsilateral pulmonary artery via the ductus arteriosus (Fig. 24.7). Depending on the patency of the ipsilateral ductus arteriosus as well as pulmonary arterial pressure, the affected subclavian artery may perfuse via several different mechanisms.
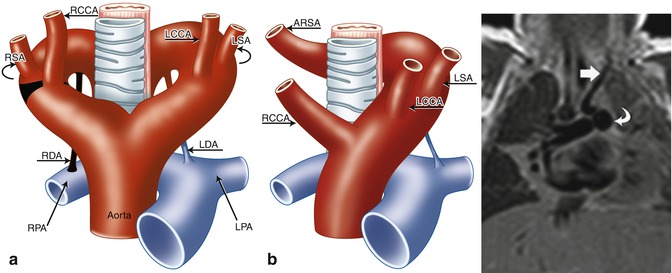

Fig. 24.7
(a, b) Schematic of right aortic arch (RAA) with isolated left subclavian artery (ILSA). (a) The areas in black represent the break points in the Edwards hypothetical double arch model. (b) Schematic of the evolved RAA with isolation of the left subclavian artery (LSA). Note the origin of the ILSA from the left pulmonary artery (LPA). (c) 1-month-old ex-28-week premature boy with double-outlet right ventricle, side-by-side great arteries (aorta to the right), subaortic ventricular septal defect, and ILSA. Coronal black-blood image shows the ILSA (straight arrow) arising anomalously from the LPA (curved arrow). LCCA left common carotid artery, RCCA right common carotid artery, RSA right subclavian artery, LDA left ductus arteriosus, RDA right ductus arteriosus, RPA right pulmonary artery
Associations
Isolation of left subclavian artery is a rare defect and occurs much more commonly with right aortic arch than left. Given that the isolated artery is generally opposite the side of the aortic arch, the lesion is typically found on the left side. In over half of cases, it is associated with intracardiac defects, the most common of which is tetralogy of Fallot [35, 36




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree