Hypertension
Genetic disorders such as Marfan and Turner syndrome, coarctation of the aorta
Inflammatory vasculitis, e.g. Behcet’s disease, Takayasu arteritis
Infective arteritis
Iatrogenic factors (cardiac valve or aortic surgery)
Pregnancy
Phaeochromocytoma, corticosteroid treatment, cocaine use
Aortic root anomalies, e.g. valvular aortic stenosis, bicuspid aortic valves
The outlook for patients depends on presentation. Patients with acute (less than 2 weeks duration of symptoms) AAS are at the greatest risk of morbidity and mortality (Abbas et al. 2014).
3 Imaging Protocol
MDCT is the gold standard imaging investigation for AAS with a sensitivity of 100 % and specificity of 98–99 % (Hiratzka et al. 2010). Although MRI and transoesophageal echo have similar sensitivity and specificity, MDCT is generally preferred in an acute setting because it is near-universally available and provides speedy acquisition and high accuracy (Salvolini et al. 2008). The ability to reconstruct in three planes and to assess aortic branches including aberrant anatomy makes CT invaluable for surgical and endovascular planning (Maddu et al. 2013; Agarwal et al. 2009). Both pre- and post-contrast CT should be obtained to differentiate between the three components of AAS (Maddu et al. 2013). Table 2 summarises the CT findings in all three entities of AAS (see later text for further explanations).
Non-contrast CT | Post-contrast CT | |
|---|---|---|
Aortic dissection | May be normal in the acute setting OR inward displacement of intimal calcification | Intimal flap, >2 lumens (TL and FL) Flow through both true and false lumens +/− true lumen compression |
Intramural haematoma | High attenuation crescentic thickening of aortic wall | Aortic wall thickening May have similar appearances to acute aortitis |
Penetrating atherosclerotic ulcer | Difficult to diagnose on unenhanced CT | Focal ulceration penetrating through aortic intima to aortic wall |
At least 64 detector rows are required to achieve multiplanar isotropic spatial resolution. ECG gating is required to reduce cardiac motion artefacts – this is particularly important in accurately diagnosing dissection at the aortic root and avoiding false positives due to cardiac motion artefact mimicking an acute dissection flap. ECG gating also improves coronary artery visualisation. The field of view for pre-contrast ECG-gated CT should extend from above the aortic arch to the inferior diaphragm. This should be acquired prospectively in mid diastole. Premedication with beta-blockers or other medications should be avoided (31 Chin in Abbas). On the non-contrast CT, high-density IMH can be identified, and local pleural or pericardial rupture can also be demonstrated (Abbas et al. 2014; Castaner et al. 2003). A bolus tracking CT angiogram achieving opacification of the aorta of >250 HU follows, covering from the thoracic inlet to the aortoiliac bifurcation or common femoral arteries, with precise timing of image acquisition and imaging parameters varying slightly with machines (Castaner et al. 2003). The usual volume of iodinated 370 mg/ml contrast medium of 120 ml is decreased in elderly patients to 80–100 ml in view of decreased cardiac output. The contrast should be injected via the right arm to avoid streak artefact from the left brachiocephalic vein near head and neck vessels. A saline flush of 20 ml further minimises streak artefact (Weininger et al. 2011). The patient is able to free breathe during abdominal aortic imaging, but chest imaging is acquired in breath-hold.
Multiplanar reformat assessment is advisable since it helps in the evaluation of the aortic root. Moreover, clinicians appreciate 2D and 3D reconstructions as they are useful for planning surgical or endovascular procedures (Agarwal et al. 2009). Maximum intensity projection reconstructions are valuable for visualisation of branch vessel involvement (Abbas et al. 2014).
Alternative techniques to MDCT in patients in whom iodinated contrast is contraindicated include transoesophageal echocardiography and MRI, but both of these techniques are time-consuming and not widely available in all hospital settings. Contrast-enhanced MR angiography has excellent spatial and contrast resolution and allows multiple vascular imaging phases and post-processing. Its limitations include the inability to demonstrate arterial wall or intimal calcification, lack of suitability for unstable patients or those with implanted electronic devices and the problem of artefacts due to stent placement (McMahon and Squirrell 2010). Initial unenhanced CT followed by MRI (where feasible) or transoesophageal echocardiography may be a reasonable compromise in patients who cannot receive iodinated contrast or in whom repeated examinations are required, as CT will demonstrate IMH, intimal calcification displacement, rupture into pleural or pericardial space and aortic size (Abbas et al. 2014).
4 Classification in Acute Aortic Syndrome: The Stanford Classification
Classification of AAS is based on the location and extension of the dissection, with the Stanford system preferred over the DeBakey system in the emergency setting because it dictates immediate clinical management (broadly speaking, surgical = type A, medical = type B) (Maddu et al. 2013). Stanford type A affects the ascending aorta or aortic arch and accounts for 75 % of aortic dissection (Castaner et al. 2003). The dissection flap may extend to the descending aorta (McMahon and Squirrell 2010). Stanford type B begins distal to the left subclavian artery. For all three entities of AAS (dissection, IMH, PAU), the diagnosis of type A involvement requires immediate referral to a cardiothoracic surgical centre.
5 Acute Aortic Dissection
There are 2000 new cases of aortic dissection each year in the USA and 3000 in Europe (McMahon and Squirrell 2010). Dissection is the most common aortic emergency, being more prevalent than thoracoabdominal aortic aneurysm rupture (Castaner et al. 2003). Acute thoracic dissection is life-threatening and requires immediate diagnosis and treatment (Castaner et al. 2003): 75 % of deaths from aortic dissection occur within 2 weeks of clinical presentation.
The normal aortic wall has three layers (from external to internal: adventitia, media and intima) (Fig. 1). The pathophysiology of aortic dissection includes disruption of the aortic intima and inner layer of the media such that blood can track along the media (Macura et al. 2003) (Fig. 2). This propagation can be both retrograde and anterograde (Maddu et al. 2013) and results in formation of a true and a false lumen with the false lumen having pressures greater than or equal to the true lumen (Williams et al. 1997). Since the intervening ‘intimal flap’ between the two lumens consists of intima and media, it should more precisely be termed an ‘intimomedial’ flap. It is theorised that reduced elastic recoil of the dissection flap enables the false lumen to dilate. This resulting collapse of the true lumen is highly variable based on the number of fenestrations and tears between the true and false lumen, the chronicity of the flap and the degree of control of the patient’s blood pressure. In our clinical experience, the patient’s response to the compressed true lumen and their overall clinical status dictates further management (McMahon and Squirrell 2010; Williams et al. 1997). The overall degree of dilation of the false lumen depends on blood pressure, residual wall thickness and percentage of wall circumference involved in the dissection. The false lumen may subsequently compress or obstruct the true lumen. The dissection may remain patent as a false lumen, may thrombose or may recommunicate with the true lumen through fenestrations (Macura et al. 2003). Alternatively, it may rupture into a pericardial or pleural space (McMahon and Squirrell 2010). The greater the proportion of the media involved in the flap, the thinner the external wall of the false lumen, and therefore, the higher the risk of rupture (Roberts 1981). The initial intimal tear occurs at sites of greatest hydraulic pressure – usually the right lateral wall of the ascending aorta or the proximal segment of the descending aorta (Maddu et al. 2013).
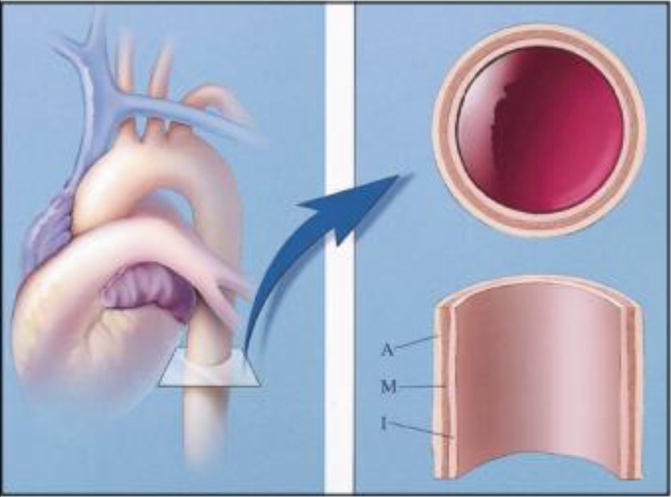


Fig. 1
Diagram shows three layers of normal aortic wall: from inner to outer, intima (I), media (M) and adventitia (A) (Reproduced from Macura et al. 2003, Pathogenesis in Acute Aortic Syndromes (Permission requested))

Fig. 2
The diagram illustrates events leading to aortic dissection from formation of entrance tear and exit tear of intima to splitting of aortic media and formation of intimomedial flap. Blood under pressure dissections medial longitudinally and double-channel aorta is formed with blood filling both true and false lumens (Reproduced from Macura et al. 2003, Pathogenesis in Acute Aortic Syndromes (Permission requested))
5.1 Imaging Features
The presence of an intimomedial flap and the double-lumen aorta are both key features of aortic dissection. Further features include the origin and extent of dissection, which is the true and which is the false lumen, and whether branch vessels are affected (Hiratzka et al. 2010). A dissection flap is identified as a thin, uniform, low-density flap on multiple contiguous slices, which is important in distinguishing it from artefactual flaps including streak artefact and cardiac motion. Streak artefacts have a variable width and orientation, radiate away from a high-density focus and may have high and low densities (Abbas et al. 2014). Cardiac motion artefact characteristically is seen in the right posterior and left anterior proximal aorta (Figs. 3 and 4).
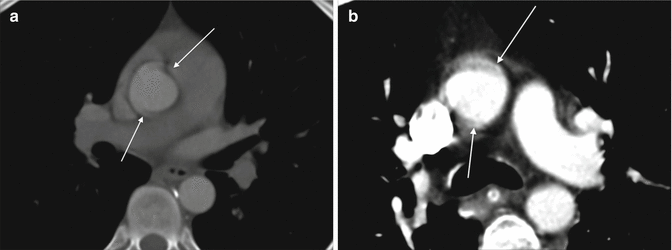
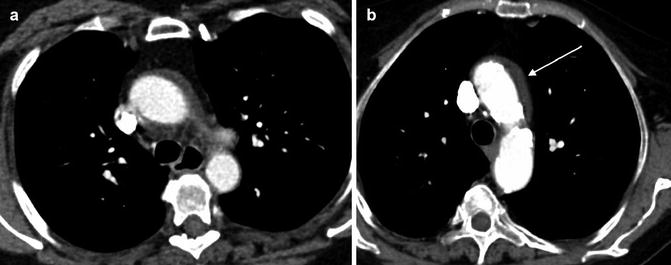

Fig. 3
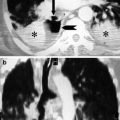
Motion artefact. A cardiac motion artefact in the ascending aorta mimics a Stanford type A dissection in 2 different patients (a and b) (arrowed)

Fig. 4
Pseudodissection. A high superior pericardial recess mimics a Stanford type A dissection (arrowed)
It is particularly important to differentiate between the false and true lumen if endovascular treatment is planned (Castaner et al. 2003). The true lumen is continuous with the non-dissected portion of the aorta and can usually be identified (Maddu et al. 2013). Differentiating between the false and true lumen is more difficult when the aortic root is involved, for instance, in rare cases of intimointimal intussusception/circumferential dissection, which produce a circumferential flap with one lumen wrapped around the other lumen in the aortic arch. The inner lumen is usually the true lumen (Castaner et al. 2003).
The false lumen can be usually differentiated from the true lumen by the presence of slower flow (lower density opacification), wider channel and presence of thin ‘cobweb’ strands due to medial tissue within the false lumen (Litmanovich et al. 2009). The ‘beak sign’ occurs at the acute angle of the intimomedial flap and outer wall of the FL. Calcification may help to differentiate between the FL and TL – it has been described as only being seen in the wall of true lumen. The ‘floating viscera’ sign occurs when the visceral arteries are opacified with contrast media, but the true lumen appears compressed (see Table 3) (Figs. 5, 6, 7 and 8).
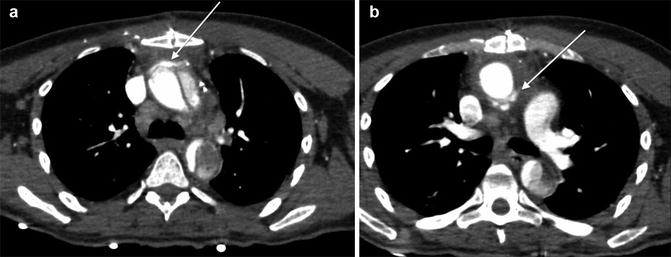
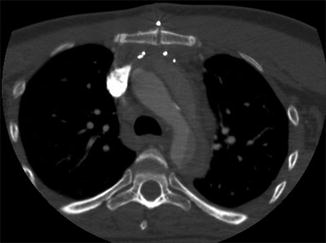
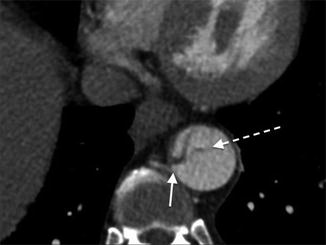
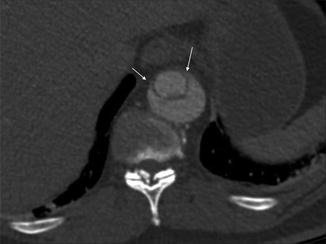
Table 3
CT findings useful in differentiating between the TL and FL
CT finding | True lumen | False lumen |
|---|---|---|
Communication with aorta | Directly communicates with aorta | Not connected to unaffected aorta |
Intimomedial flap rupture | Communicates with false lumen | |
Calibre | Smaller than FL | Larger than TL |
Intimal flap | Calcified | Surface of flap is convex |
Enhancement | Enhances more than false lumen | Slower flow therefore hypodense to TL (in the arterial phase) |
Other signs | Cobweb sign Beak sign ‘Floating viscera’ sign (true lumen is compressed but contrast is seen in visceral arteries) |

Fig. 5
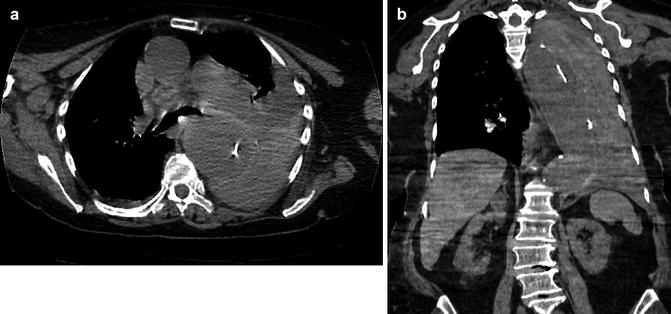
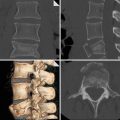
Stanford type A dissection. Note extravasation of contrast from ascending aorta giving rise to mediastinal haematoma (arrowed)

Fig. 6
Stanford type A aortic dissection. Note displacement of the intimal calcification by clot in the false lumen which lies lateral to the intimal flap

Fig. 7
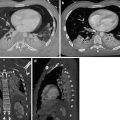
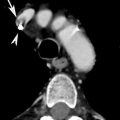
Stanford type B dissection. Note the cobweb sign (dotted arrow) present which is caused by strands of fragmented media in the false lumen. Although not commonly seen, the cobweb sign is highly specific for the false lumen. Note the beak sign (solid arrow) caused by the false lumen as it contacts the junction of the intimal flap and aortic wall

Fig. 8
The beak sign. The false lumen forms (arrowed) an acute angle with the aortic wall and the intimal flap. The true lumen is circular in cross-section; the false lumen is crescenteric
Dissection with a thrombosed false lumen can be difficult to differentiate from aortic aneurysm with intraluminal thrombus, but the dissection will generally have a spiral shape, whereas the aneurysm and thrombus tend to maintain a constant circumferential relationship with the aortic wall. The mural thrombus usually has an irregular internal border, whereas dissection has a smooth internal border. Finally, intimal calcification is peripheral in an aortic aneurysm (Castaner et al. 2003).
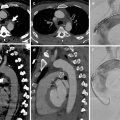
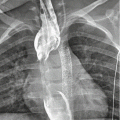
Complications of dissection include pericardial tamponade, acute aortic regurgitation, aortic rupture and major vessel occlusion (Hiratzka et al. 2010) (Fig. 9). Rupture is more common in ascending aortic dissection with rupture into the pericardium, left pleural cavity or mediastinum; hence, the treatment of ascending aorta dissection is always surgical (Abbas et al. 2014; Castaner et al. 2003). The radiologist can help the surgeon by assessing involvement of the coronary arteries in very proximal aortic dissection (Chiu et al. 2013). The risk of fatal aortic rupture in untreated proximal aortic dissection is 90 % with 75 % of ruptures taking place in the pericardium, left pleural cavity or mediastinum, where hyper-attenuating fluid will be demonstrated on unenhanced CT with extravasation on contrast-enhanced CT (Castaner et al. 2003). The presence of a pericardial effusion is a particularly ominous sign, suggesting a rupture or leak (Castaner et al. 2003) (Fig. 10). A rarer complication of type A dissection is mediastinal haematoma dissecting the sheath of the pulmonary arteries due to blood flow from the ascending aorta to the interstitial space around the pulmonary arteries (Castaner et al. 2003) (Fig. 11).