Investigators
Year
Patients
Graft no.
Sensitivity
Specificity
FD. Rubens
2002
20
–
–
–
O. Reuthebuch
2003
38
124
–
–
DP. Taggart
2003
84
213
–
–
L. Balacumaraswami
2004
200
533
–
–
M. Takahashi
2004
72
290
–
–
ND. Desai
2006
46
139
83.3
100
T. Handa
2009
39
116
100
100
T. Handa
2010
51
129
85.7
100
Here we report our experience with bypass evaluation by HEMS angiography during CABG and peripheral arterial bypass grafting in cardiovascular surgery [8, 12, 13]. Furthermore, we introduce an appraisal method to evaluate intestinal blood flow for patients with acute intestinal ischemia [12].
7.2 Usefulness of HEMS Angiography in CABG
Off-pump coronary artery bypass grafting (OPCAB) was established as standard technique of CABG for treatment of angina patients, which has lowered the incidence of cerebral infarction and reduced operative mortality about 0.6 % [14]. On the other hand, OPCAB maintains heart rhythm for anastomosis during bypass of the coronary arteries. Moreover, the anastomotic site is displaced downward to the distal side of the coronary artery in OPCAB, compared to cardiac arrest in conventional CABG [15]. The difficulty of CABG is due to these two factors. As a result, a decrease in bypass patency in OPCAB and worsening of bypass anastomosis as an early operative result have been reported [15]. By evaluating bypass flow and the anastomotic state during surgery, surgeons are able to reanastomose the bypass intraoperatively. Such an approach was required by risk management and further clinical training of cardiovascular surgeons. Various types of flowmeters, echography systems, and X-ray coronary angiography (CAG) using a catheter have been used to evaluate bypass grafting during surgery. The transit time flowmeter (TTF) to evaluate the bypass graft by measuring the flow volume and flow pattern has been reported that noted the sensitivity and specificity of the device [16]. Even if there is a lot of flow volume of blood, it does not reflect the state of its anastomotic site. Although the anastomotic site can be visualized during CABG, manipulation of the catheter can be cumbersome during surgery, and X-ray imaging can be difficult to employ during surgery in a general hospital setting. The ICG angiography system using ICG fluorescence was developed to increase the precision of the bypass appraisal method by visualization of bypass flow in CAG to better describe the anastomosis. Although, several types of ICG imaging devices are commercially available, the HEMS angiography has two distinct advantages [7, 8, 13]. The first is visualization of blood flow, which is shown as ICG fluorescence of the bypass graft displayed on the monitor of the HEMS. Blood flow appears as fluorescent white in the bypass graft against a colored background of the surgical field on the monitor. As a second advantage, excitation light is generated from a light-emitting diode (LED), as opposed to a medical-grade laser; thus, the imaging time is unrestricted with the HEMS. An assessment method to describe and evaluate myocardial perfusion flow during CABG has also been reported [17]. Because an observation time of 30 s or more is required, this procedure by HEMS angiography is considered useful [7, 8, 13].
7.2.1 Setup and Imaging by HEMS Angiography for CABG
We recently developed the HEMS to resolve some problems with current ICG imaging devices [13]. The HEMS consists of an imaging unit, control unit, and monitor. The imaging unit consists of multiple LEDs around an ultrasensitive color charged-coupled device (CCD) imaging camera with non-Bayer color filter arrays (HyperEye Technology; SANYO Co., Ltd, Tokyo, Japan), which can detect near-infrared rays (380–1200 nm) and visible light at 30 frames/s at a time. The control unit consists of a computer and controller to record and adjust the focus and iris. The HEMS employs Diagnogreen (Daiichi Sankyo, Tokyo, Japan) as the ICG dye, which is prepared with 2.5 mg/mL dissolved in distilled water [8, 12]. The imaging head is draped by a sterile cover and placed 30–50 cm above the target (Fig. 7.1A). The LED illumination area is approximately 78.5 cm2 on the surgical field. Conventionally, the standard dose of ICG solution is 5 mg per imaging sequence. The ICG solution is intravenously injected through a central or peripheral venous catheter and flushed with 10 mL of physiological saline (Fig. 7.1B). This allergy reaction has been reported to occur at an incidence of an approximately 1:40,000. The fluorescence of arterial blood flow is displayed on the monitor and recorded using a digital image-processing system with an audio video interweave or SmartDraw format.
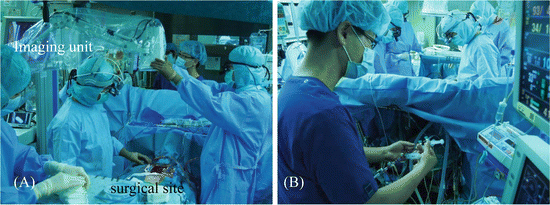

Fig. 7.1
HyperEye Medical System (HEMS). (A) The imaging head is draped by a sterile cover and placed 30–50 cm above the target. (B) ICG solution is injected through a central or peripheral venous catheter. ICG indocyanine green
7.2.2 Evaluation of Coronary Artery Bypass Patency by HEMS Angiography
The bypass graft of an anastomotic stenosis has a time lag from imaging of the graft to the distal side of the coronary artery. The HEMS angiographic images are classified into two categories, normal and abnormal opacification, with the latter subclassified into two categories, as follows [8]:
Normal: smooth opacification of the graft, coronary artery, and myocardium (Fig. 7.2A)
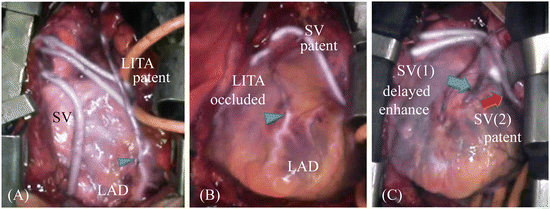
Fig. 7.2
The HEMS angiographic images are classified into three categories: (A) normal, smooth opacification of the graft and coronary artery; (B) occlusion, no enhancement of the graft; and (C) delay, delayed graft enhancement compared with other grafts. Arrow head shows anastomoses. Arrow showed the delayed enhanced bypass graft (blue arrow) and patent graft (red arrow)
Abnormal: delayed or inadequate opacification of the graft
Occlusion: no enhancement of the graft (Fig. 7.2B)
Delay: delayed graft enhancement compared with other grafts (Fig. 7.2C)
Resolution of the HEMS angiograph showing arterial dissection of the bypass can distinguish a true lumen from a pseudo lumen. Although the flow competition from the coronary artery was classified as abnormal, it was considered an exception to these criteria because it was not a problem associated with anastomosis.
7.2.3 Pitfalls of HEMS Angiography
The setup and use of HEMS angiography are relatively simple, and the passage of blood flow can be observed with the monitoring screen; however, experience of HEMS is essential. Therefore, the surgeon should have knowledge of the properties of ICG and the features of each component of the ICG angiography apparatus. Several points to consider in the evaluation of HEMS angiography are discussed below.
7.2.3.1 Penetration Depth and Fluorescent Luminescence
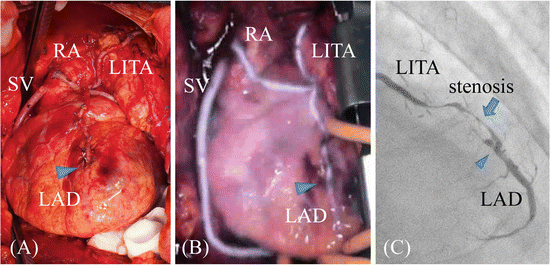
There are differences between HEMS angiography and X-ray CAG because an X-ray passes through the human body to show an anastomotic site of the coronary artery, whereas ICG fluorescence is unable to pass through the thoracic wall; thus, its actual permeable depth is approximately 2–3 mm [13]. The accuracy of ICG angiography to estimate the degree of stricture of the lumen at an anastomotic site is limited. Moreover, ICG solution adheres to the vessel walls as it passes through the bypass grafts. Therefore, although the bypass and anastomotic site are imaged, only the vessel outline of the bypass graft can be observed. Figure 7.3 shows an image of three bypassed branches following OPCAB (left internal thoracic artery (LITA)/left anterior descending artery (LAD), radial artery/posterolateral branch, and greater saphenous vein (SV)/right posterior descending branch of a 69-year-old man with unstable angina) (Fig. 7.3A). The HEMS angiography showed no stenosis of anastomosis and bypass graft with smooth opacification (Fig. 7.3B). Though, X-ray CAG in the early postoperative stage showed that the distal end of the graft had a diffuse stricture and that blood flow was obstructed (Fig. 7.3C). In a review of HEMS angiography results, the rate of blood flow through the LITA was decreased in this case. However, although blood flowed through the entire graft, we were unable to identify the stricture by HEMS angiography. Therefore, we have developed a qualitative method to evaluate the time lag of flow passage [8, 13]. If ICG imaging of the bypass has delayed enhancement, it can be estimated with a delayed image called category “delay” (Fig. 7.2C). Fat deposits reflect ICG fluorescence; therefore, if the fat layer covers the arterial surface, it disrupts flow evaluation. Thus, although the ICG fluid adheres to the anastomotic surface, permeability is decreased. When the coronary artery, which is buried in the myocardium, and fat layer are thick, native coronary arterial blood flow cannot be visualized but is rather judged by influent and washout of ICG fluorescence passing through the grafts. Because the distal side of the LAD isn’t often buried in the ventricular wall, the patency was evaluated by smoothness of flow from the ITA graft to LAD. On the other hand, it is often difficult to navigate the coronary artery downward. At the time of reoperation, the coronary artery is unobservable from the heart surface because of scar tissue on the coronary arteries. Although we examined the usefulness of angiography using the HEMS to navigate the target coronary artery during CABG, the ability to identify the coronary artery is decreased compared with that of echography of the cardiac surface because of its low ICG permeability. However, the coronary veins are thick with thin walls and run more superficially within the heart. Even when the coronary veins are covered with a scar layer, they can be visualized by HEMS angiography. In addition, the target coronary artery can be identified by contrasting the coronary vein described by the HEMS angiography with the coronary arteries identified by computed tomography and CAG before surgery. Furthermore, Shikayama reported that the penetration depth of the PDE imaging system is approximately 10 mm. The PDE system is maybe effective for observations at limited depths.