8 Arterial Disease Peripheral artery disease (PAD) refers to progressive narrowing of the aorta and the major arteries of the extremities and organs, essentially the body’s entire circulation except the brain and heart. PAD is almost always due to atherosclerosis, which is cholesterol and inflammatory cell buildup in the vessel wall. While less common, other causes of PAD include emboli, extrinsic compression, trauma, adventitial cystic disease, peripheral aneurysms, and vasculitis. As atherosclerosis is a systemic condition, patients with PAD also commonly have coronary artery and cerebrovascular disease. The long-term cardiovascular morbidity and mortality in these patients is high, and much of the medical management strategy is centered around reducing overall cardiovascular risk. The most commonly affected vessels in PAD are those of the lower extremity, with nearly half of all PAD patients experiencing symptoms from the disease. Management of the symptomatic PAD patient differs from that of the asymptomatic patient. Asymptomatic PAD may be diagnosed in the outpatient setting, often by a primary care physician or cardiologist. Given the number of comorbid conditions associated with PAD, it should be highly suspected in smokers, diabetics, patients older than 50 (especially males), and those with hypertension, hyperlipidemia, coronary artery disease, or prior stroke. Making the diagnosis is important, even in asymptomatic patients. Having PAD is an indicator of high risk for vascular disease throughout the body, and initiation of appropriate medical therapy in an asymptomatic patient could prevent a heart attack or stroke. Patients with risk factors should be initially screened for PAD with a thorough history. It’s important to ask about leg pain with exercise, leg pain at rest, and the presence of nonhealing wounds. On physical exam, you want to check for pulse strength and bruits throughout the body. A detailed visual inspection of the legs and feet is important. Any nonhealing ulceration, skin discoloration, or gangrene may signify advanced disease. Keep in mind, a patient may have a normal exam in the legs, but evidence of vascular disease elsewhere. You need to listen for carotid bruits, measure blood pressure in both arms (a difference between the two may be a clue to subclavian stenosis), and palpate the abdominal aorta. Patients with suspected PAD can be further evaluated with a resting ankle-brachial index (ABI) (▸Table 8.1). The test is basically a calculation of the ratio of the ankle blood pressures in each leg to the brachial pressures in each arm. An ABI less than 0.9 is consistent with PAD, but a normal value doesn’t rule it out. If suspicion remains high in patients with a normal or borderline ABI, they are further evaluated with an exercise ABI. This is especially useful to differentiate PAD from the other types of exertional extremity pain (osteoarthritis, spinal stenosis, venous hypertension). An exercise ABI with 15% or greater decrease compared to the pre-exercise value is abnormal and consistent with PAD. Table 8.1 Ankle–brachial index
8.1 Peripheral Artery Disease
Approach to the Asymptomatic Patient with Suspected PAD
ABI | Interpretation |
> 1.3 | Falsely high value |
0.9–1.3 | Normal |
0.7–0.9 | Mild PAD |
0.4–0.7 | Moderate PAD |
< 0.4 | Severe PAD |
Abbreviation: PAD, peripheral artery disease. | |
Falsely elevated ABIs (> 1.3) are common in patients with chronic kidney disease and diabetes, related to calcification causing noncompressibility of the artery. If this is seen, the ABI should be supplemented with a toe-brachial index (TBI). The vessels of the great toe are often spared, even in the setting of severe calcification more proximally. A TBI of less than 0.7 is diagnostic of PAD.
Approach to the Symptomatic Patient with Suspected PAD
When a patient presents with lower extremity pain, keep in mind the nonvascular etiologies of leg pain (▸Table 8.2). Symptoms of PAD range from mild to debilitating, often described as cramping or aching. Pain is caused by nerve and muscle ischemia, affecting muscle groups distal to the level of flow restriction (calf pain due to femoral–popliteal occlusions; buttock and thigh pain from aortoiliac occlusions, etc.). Symptomatic PAD presents in one of three ways: intermittent claudication, atypical leg pain, or critical limb ischemia. Vascular specialists commonly use the Rutherford scale to stratify symptomatic patients (▸Table 8.3).
Intermittent claudication (IC) is the mildest form of symptomatic PAD, comprising stages 1 to 3 on the Rutherford scale. It is characterized by extremity pain that occurs after walking a certain distance and is relieved with rest. The distance to symptom onset is consistent and reproducible but can be shortened when walking uphill or going up stairs.
Atypical leg pain is related to IC but occurs when patients do not have the classic intermittent claudication symptoms. Rather, they may have pain after walking that isn’t debilitating enough to stop walking, or other nonspecific lower extremity symptoms. This is the catch-all category of lower extremity symptoms that don’t neatly fit the bill of claudication. Thus, even if the patient doesn’t have a classic story for claudication, be aware that PAD can present with atypical leg symptoms.
Critical limb ischemia (CLI), comprising stages 4 to 6 on the Rutherford scale, is characterized by pain at rest, ulceration of the skin, nonhealing wounds, and/or gangrene. While intermittent claudication and critical limb ischemia are both variants of PAD, only the latter is limb-threatening.
Symptomatic patients with suspected peripheral vascular disease will get an ABI. When critical limb ischemia is suspected, a number of studies are done together to confirm the diagnosis and localize the atherosclerotic burden for treatment planning. Ultrasound is often used to locate and gauge severity of the disease. Normal triphasic Doppler waveforms will change to damped monophasic waveforms beyond the level of a significant stenosis or occlusion. Additional tests include segmental pressure measurements, pulse volume recordings (PVRs), transcutaneous oxygen pressure measurements (TcPO2) and skin perfusion pressure (SPP). Each of these tests is designed to help delineate the level of disease or distinguish between ischemic and nonischemic ulceration. They can also help predict the likelihood of wound healing after intervention. A full discussion of these tests is beyond the scope of this book.
Table 8.2 Differential diagnosis for lower extremity pain and differentiating features
Etiologies of lower extremity pain | Differentiating features |
Neurogenic pain | Sharp pain that radiates down the leg; pain can be reproduced/alleviated with maneuvers that change back position |
Musculoskeletal pain | Pain over muscles and joints, usually accompanied with tenderness to palpation; pain occurs with activity |
Pain due to venous disease | Pain, “heaviness,” “tightness” in entire leg after walking; pain relieved with elevation of the leg |
Claudication | Leg muscle pain that is reproducible with walking certain distances; pain relieved quickly with rest |
Table 8.3 Rutherford’s classification for chronic limb ischemia
Stage 0 | Asymptomatic |
Stage 1 | Mild claudication |
Stage 2 | Moderate claudication |
Stage 3 | Severe claudication |
Stage 4 | Pain at rest |
Stage 5 | Tissue loss/ulceration |
Stage 6 | Extensive tissue loss/ulceration/gangrene |
Some clinicians rely on CT angiography as it tends to be a quick test to stratify location and burden of disease in the various segments of the peripheral arterial bed, including aortoiliac, femoral–popliteal, and tibial–pedal disease.
Management of PAD
Medical management is extremely important for all patients with PAD, regardless of the Rutherford class. Patients are put on an antiplatelet agent, either aspirin or clopidogrel, and a moderate- or high-intensity statin. Hypertension and diabetes should be controlled according to established guidelines, and smoking cessation strongly encouraged. Optimized medical therapy and lifestyle modification can slow the progression of atherosclerotic disease and help to prevent future cardiovascular events.
The diagnosis of PAD in an asymptomatic patient is a warning sign, alerting us to atherosclerotic disease likely present throughout the patient’s body. If peripheral arteries are affected, coronary, carotid, renal, and mesenteric arteries may be affected as well. All PAD patients should undergo screening for subclavian stenosis, carotid stenosis, and abdominal aortic aneurysm (AAA), regardless of age, gender, or smoking status.
In symptomatic patients with intermittent claudication only, the management goals include symptom control, improvement in ambulatory status, and cardiovascular risk reduction. Because intermittent claudication is not limb-threatening, patients can be managed on an outpatient basis. After initiating optimal medical therapy, most patients will benefit from supervised exercise therapy. This involves participation in 30 minutes of walking, typically at least three times a week. During the exercise, patients walk to the point of maximum claudication (and just beyond, if possible), rest until the pain abates, and then repeat. Only walking time is counted toward the 30-minute goal. The CLEVER trial showed that participation in supervised exercise therapy improved pain-free walking distances comparable to treatment with endovascular therapy.
Exercise programs have excellent long-term durability and the added benefit of improving overall cardiovascular health. The initial trial of exercise should last around 3 months before symptoms are reassessed. While many patients were previously forced to perform this program at home due to lack of coverage by insurance companies, supervised exercise therapy is now being covered by Medicare and most insurance providers.
If symptoms persist despite exercise therapy, the patient may be started on cilostazol, a phosphodiesterase inhibitor. Cilostazol has both antiplatelet and vasodilatory effects. It has been shown to increase pain-free walking distances, but does not affect progression of the disease. Many patients are intolerant due to side effects (headache, diarrhea, dizziness, palpitations), and it is contraindicated in those with heart failure.
Most patients with claudication see considerable improvement with conservative therapy. The minority who continue to have lifestyle-limiting claudication despite an adequate trial of conservative therapy may be candidates for an invasive intervention. Diabetic patients tend not to improve as quickly or as completely as their nondiabetic counterparts, and more frequently require an intervention to achieve an acceptable level of symptomatic improvement.
For patients with critical limb ischemia (rest pain, ulcerative skin changes, nonhealing wounds, gangrene), the disease has progressed to the point of becoming limb-threatening. The goal of therapy for these patients is, first and foremost, limb salvage. Early intervention and revascularization can reduce tissue loss, relieve rest pain, and allow for existing wounds to heal. If limb salvage is not possible or fails, amputation may be necessary.
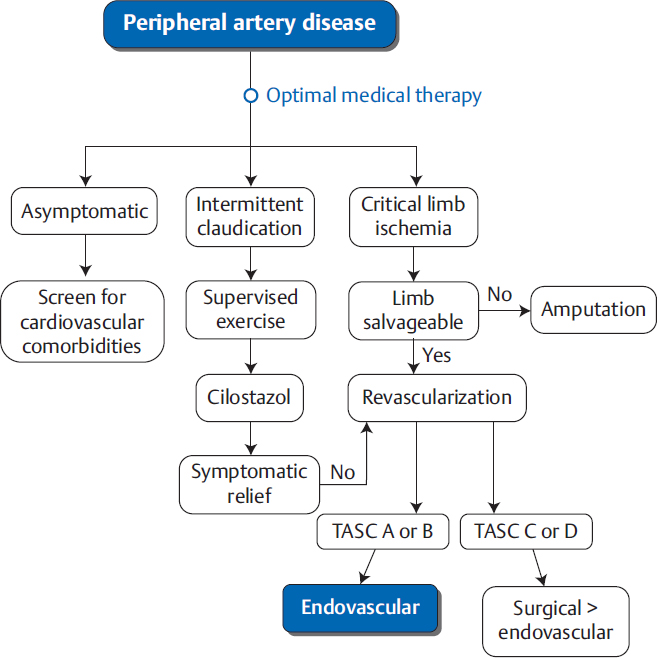
When there is an indication to revascularize an extremity, vascular specialists have two options: surgery or endovascular procedures (▸Fig. 8.1). Deciding which treatment method is most appropriate can be a complicated decision, and is often influenced by the experience of the operator.
Surgical options for treating PAD include endarterectomy and bypass. Endarterectomy involves surgical exposure of the diseased arterial segment and physical removal of the thrombosed plaque through an arteriotomy. This is often combined with patch angioplasty, which includes sewing a bioprosthetic or artificial patch onto the arteriotomy site during closure to widen the lumen and accommodate for postsurgical scarring. Bypass involves surgically suturing the proximal portion of a conduit (either a vein or a synthetic graft) upstream to the diseased segment of the artery, and suturing the distal portion of the conduit downstream of the diseased segment, effectively rerouting blood past the diseased arterial segment. Common bypasses you will see include aortofemoral/aortobifemoral, axillofemoral, femoral–femoral, femoral–popliteal, and femoral–tibioperoneal.
To maximize graft patency, the proximal and distal anastomosis sites should be disease-free segments, allowing for adequate inflow and outflow from the graft. Autologous veins, often harvested greater saphenous veins, tend to have much higher patency rates than synthetic grafts (polytetrafluoroethylene [PTFE]). Whenever possible, prebypass vein mapping should be performed to determine the quality of veins available for harvest.
Fig. 8.1 Simplified algorithm for management of peripheral arterial disea. Note that many TASC C and D lesions are now being treated endovascularly. The decision to proceed with surgical revascularization over endovascular revascularization takes into account the patient’s comorbidities, surgical risk, and the lesion characteristics.
Endovascular treatment for PAD has been used since the 1960s as a means of opening up arteries and increasing downstream flow. This typically involves some combination of angioplasty, stenting, and/or atherectomy. As a general rule, endovascular treatments are most successful in larger arteries with focal stenoses. In the setting of multilevel disease in claudicants, treating aortoiliac disease alone is often sufficient for symptomatic improvement. The procedure employed depends on the location, length of diseased segment, and the presence or absence of calcification.
Specialty wires, new device technology, and novel techniques have allowed interventional radiologists to treat arterial occlusions that previously would not have been amenable to endovascular therapy. Previously “uncrossable” occlusions have become treatable due to increased comfort and expertise with tibial and pedal retrograde access. An advanced technique aided by new technology involves crossing arterial lesions by guiding a wire in the subintimal space just proximal to the lesion and dissecting alongside the lesion. Once past the occluded segment, reentry devices can be used to regain access from the subintimal space back into the native arterial lumen, at which point a stent is typically deployed across the entire diseased segment. In addition, drug-eluting technology on balloons and stents have improved the patency of endovascular interventions.
Endovascular Interventions versus Surgery for PAD
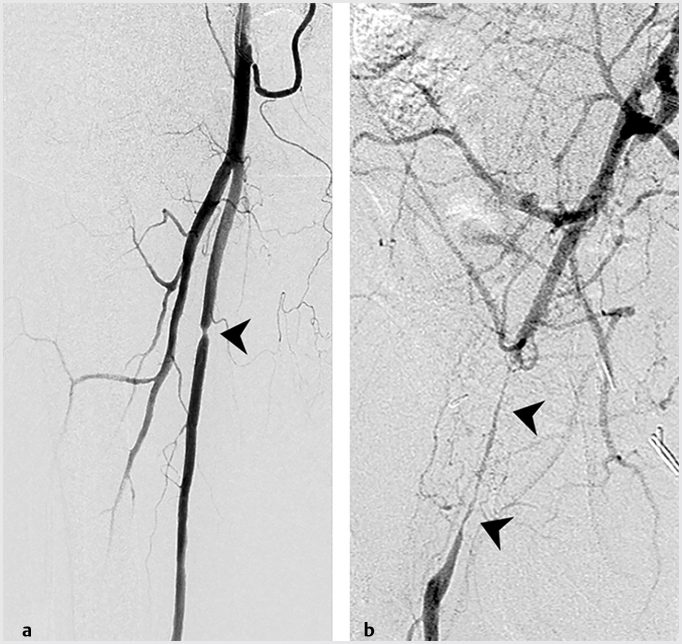
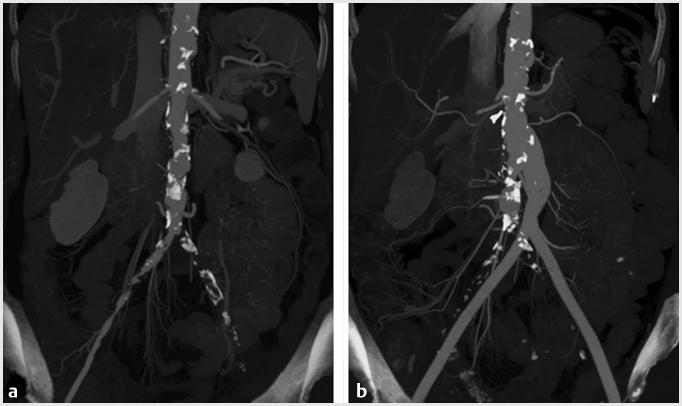
The Transatlantic Inter-Society Consensus (TASC II) guidelines classify patients according to lesion location and burden. TASC breaks down lower extremity PAD into three subsets: aortoiliac (inflow), femoral–popliteal (outflow), or tibioperoneal (runoff) disease. Disease severity and complexity is then graded for each subset on a four-point scale, from A to D. Here’s a simplified way of thinking about TASC lesions. TASC A and B disease includes short-segment stenoses or occlusions, unilateral or bilateral (▸Fig. 8.2). TASC C lesions tend to be a little longer. TASC D disease covers diffuse, multifocal, or large-vessel stenoses/occlusions (▸Fig. 8.3).
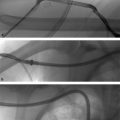
Fig. 8.2 (a) Angiographic images demonstrating a short TASC A stenosis involving the superficial femoral artery and (b) a longer TASC B stenosis involving the common femoral artery. (These images are provided courtesy of Matthew Czar Taon, MD, Kaiser Permanente Los Angeles.)
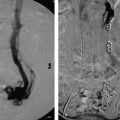
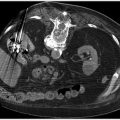
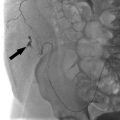
Fig. 8.3 (a) CT of the abdomen and pelvis showing extensive aortoiliac atherosclerotic disease consistent with a TASC D lesion. (b) This lesion was treated with an aortobifemoral bypass. (These images are provided courtesy of Matthew Czar Taon, MD, Kaiser Permanente Los Angeles.)
TASC II classification is a guide to the complexity of a lesion based on lesion length, location, and involved vasculature, which can be an aid in determining the technical difficulty of endovascular revascularization.
For all subsets (aortoiliac, femoral–popliteal, or tibioperoneal disease), endovascular therapy is considered first line for TASC A and B disease. Surgery is often favored in patients with TASC C and D disease (▸Fig. 8.3). It is important to note that as technology and experience with endovascular therapy has improved, the trend has been toward increasing use of endovascular techniques for TASC C and even TASC D lesions, particularly in patients with poor surgical candidacy. In the hands of a skilled interventionalist, endovascular treatment is technically feasible in all but the most recalcitrant lesions.
Given these advances, many endovascular specialists have embraced an endovascular-first approach to PAD cases. Many vascular patients tend to have multiple medical comorbidities which make them poor surgical candidates; in these patients, a less invasive approach is preferred. If surgery is being considered, the adequacy of the autologous vein conduit should be evaluated.
In addition to disease burden, the artery involved may determine if surgery is favored over endovascular therapy. For example, many vascular specialists prefer treating common femoral artery disease surgically, given the relatively straightforward surgical approach to this area, and the risks associated with endovascular treatment, including stent fracture or dissection. Similarly, popliteal artery disease is also more commonly treated with surgical bypass given its location and the risk of stent malfunction when deployed across a joint.
Another important consideration is the patient’s overall prognosis and expected lifespan. Findings from the BASIL trial (2005) suggested that for patients with longer expected life-spans, surgical bypass should be preferred. Despite the increased morbidity associated with bypass surgery, patients expected to live longer may benefit most from the higher patency rate of a bypass. For those with shorter expected lifespans, endovascular stenting is preferred.
8.2 Acute Limb Ischemia
The natural history of peripheral artery atherosclerotic disease is a gradual narrowing of the arteries, with progressive symptom severity corresponding to the degree of luminal stenosis. Ischemia can, however, develop rapidly when an artery becomes acutely occluded. This is referred to as acute limb ischemia (ALI). There is often a very high risk of limb loss with ALI if not treated immediately, as many patients lack the collateral blood supply seen in those with chronic arterial occlusions.
Work-up of a Patient with Acute Limb Ischemia
ALI must be included in the differential for all patients presenting with acute limb pain. The classic signs of ALI include the 6 P’s: pain, pallor, pulselessness, paresthesias, poikilothermia (cool to touch), and paralysis. Pain is typically the earliest and most consistent sign, while the others may be more variable.
There are two major causes of ALI: in situ thrombosis and occlusion due to emboli. Management is different for each.
Patients with known peripheral vascular disease presenting with acute limb ischemia most likely have in situ thrombosis. This type of occlusion is caused by rupture of an unstable atherosclerotic plaque, with rapid accumulation of clot leading to vessel obstruction (analogous to how coronary artery disease leads to myocardial infarction [MI]).
Those presenting with ALI and no history of PAD should be strongly suspected to have an embolic occlusion, especially when they have no PAD risk factors and good pulses in the contralateral limb. A cardiac source (from atrial fibrillation) is most typical, but embolus can also originate from aortic or peripheral artery aneurysms. An embolus travels downstream in the artery and lodges itself, usually at a branch point.
ALI is a clinical diagnosis. Treatment should be initiated based on history and physical exam alone, though an ABI can confirm the diagnosis if necessary. The blood pressure reading below the level of the occlusion is usually zero. Many clinicians use arterial Doppler to identify the location of the occlusion and determine the urgency of intervention. CTA, if time permits, can also confirm the location and be used to guide therapy.
Management of a Patient with Acute Limb Ischemia
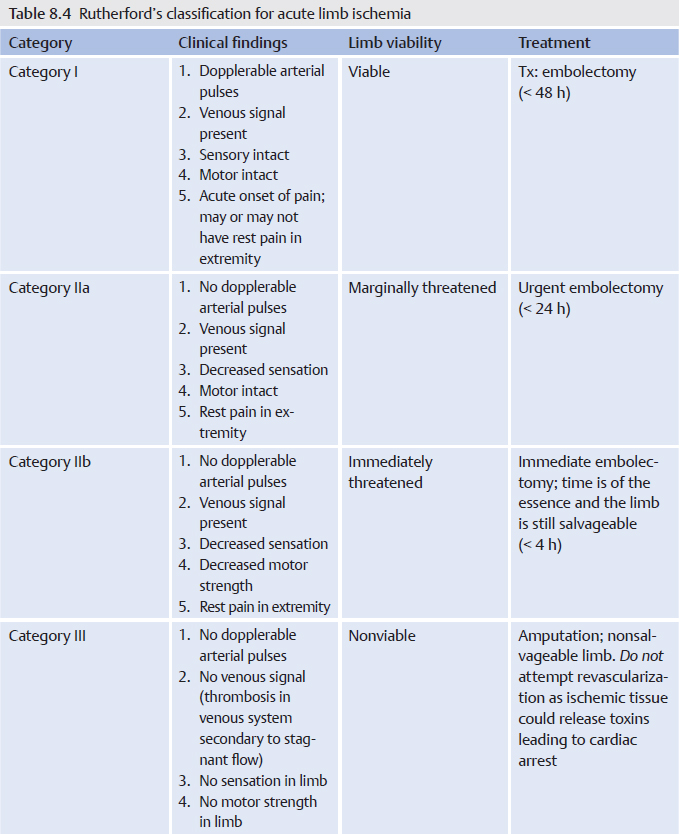
All patients with acute limb ischemia should be immediately started on a heparin drip. Anticoagulation decreases propagation of clot and worsening of limb ischemia. The urgency of additional treatment is based on the extent of damage to the limb. The vascular and peripheral neurologic exam is crucial in determining whether the limb is viable, threatened, or nonviable. Determining this is the most important initial step once ALI is suspected. This is categorized by the Rutherford classification for acute limb ischemia (▸Table 8.4), which is distinct from the Rutherford classification for chronic disease.
Nerves are more sensitive to ischemia (irreversible damage within 6–8 hours), while muscle can tolerate hypoxia for longer periods. For this reason, sensory loss will always precede motor deficits. Venous Doppler signals are usually detectable, even in the absence of arterial signal, up until the point of profound ischemic damage. The presence of both sensory and motor deficits, as well as the loss of both arterial and venous Doppler signals indicates irreversible damage.
Determining the viability of the limb (viable, threatened, or nonviable) dictates the urgency of the intervention and type of intervention. According to the Rutherford classification, viable limbs (categories I and IIa) require invasive intervention, but not emergently. This can be managed by both surgical and endovascular treatments. With category IIb, the limb is imminently threatened and requires immediate surgical revascularization. Category III limbs are considered nonviable; revascularization could actually lead to the release of toxins from the dead tissue into the blood and increases the risk of cardiac arrest. Amputation is often the only solution.
Surgical options include embolectomy for embolic occlusions, and endarterectomy or bypass for in situ thrombosis. Surgical embolectomy involves a cutdown to the artery proximal to the occlusion, insertion of a catheter through a small hole in the artery and advancing it across the clot. Once across, a balloon at the tip is inflated and then the catheter is retraced, pulling the clot out with it. In contrast, endarterectomy involves exposure of the thrombosed artery and physical removal of the thrombosed plaque through a larger incision. If the plaque burden is too great, a bypass may be the only option. In reality, these techniques are often used in combination for many patients.
Endovascular treatments include catheter-directed thrombolysis and mechanical thrombectomy (Procedure Box 8.1, Procedure Box 8.2). Catheter-directed thrombolysis involves guiding a catheter into the clot and infusing thrombolytics, usually tissue plasminogen activator (tPA). It is best suited for fresh thrombus rather than chronic, organized clots (including chronic embolized material) (▸Fig. 8.4).
The TOPAS trial (Thrombolysis or Peripheral Artery Surgery) and the STILE trial (Thrombolysis for Ischemia of the Lower Extremity) both showed that for viable limbs (category I and IIa acute limb ischemia), lytic therapy is safe and effective. Thrombolysis has the advantage of lysing smaller, more distal thrombi in addition to the occlusive thrombus. As is the case anytime a thrombolytic is used, the major contraindications include active bleeding, and recent history of stroke or surgery.
Mechanical thrombectomy involves endovascular disruption and removal of the clot. Given the risk of downstream embolization, this has historically been done less frequently than catheter-directed thrombolysis, however, newer tools and embolic protection devices have increased interest in mechanical thrombectomy as a viable treatment option for ALI.
The main advantages of endovascular treatments over surgical treatments are shorter recovery time and the avoidance of general anesthesia (important since most of these patients have significant cardiopulmonary comorbidities).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree