10 Dialysis Access and Interventions A baseline knowledge of the indications for dialysis is a good starting point for understanding how IR is involved in the care of these patients. Short-term dialysis in the acute setting and permanent dialysis for kidney failure both achieve the same goal, which is either augmentation or complete replacement of inadequate kidney function. Acutely, the main indications for dialysis include severe acidosis, electrolyte abnormalities, fluid overload, dialyzable toxemias (such as lithium or aspirin overdose), and uremia. Determining whether the problem is severe enough to initiate dialysis is typically determined by the consultant nephrologist. If the problem is believed to be reversible, a temporary hemodialysis (HD) line for access will often suffice. In most cases these are simply placed at the bedside by the clinical service taking care of the patient. IR does not put temporary lines in unless there have been multiple failed attempts (which may indicate complex/altered venous anatomy or central venous occlusion). When there is some indication at the outset that the patient will need access for longer than a week, IR may be asked to place a tunneled hemodialysis line. Chronic kidney disease (CKD) is common and often asymptomatic in earlier stages, with hypertension and diabetes being the two biggest risk factors for disease progression. Prudent medical management of these comorbidities can prevent or at least slow a decline in kidney function. The glomerular filtration rate (GFR) is the best overall assessment for evaluating CKD, and is estimated using serum creatinine. A GFR less than 60 for at least 3 months defines CKD. In earlier stages, CKD can be managed by the primary care physician. Patients have their GFR and urine albumin checked at regular intervals to monitor for disease progression. Referral to nephrology is indicated when GFR is less than 30 or when there is rapid progression. Kidney failure is defined by a GFR less than 15 with symptoms or other specific criteria that would necessitate initiation of chronic renal replacement therapy. Note that patients can be labeled as having kidney failure when they reach this threshold, but this does not necessarily indicate dialysis is imminent. The designation of end-stage renal disease (ESRD) refers to the requirements that are met in order to qualify for Medicare coverage of renal replacement therapy. The timing of initiating dialysis has many factors that go into it, but generally speaking the process is started only when symptoms of uremia arise, and with a GFR typically below 12. Transplant is an option for selected kidney failure candidates, but for our purposes we’ll focus on the role of dialysis. Options include peritoneal dialysis (PD) and HD. There are several types of peritoneal dialysis, but the concept is similar for each; dialysis solution is infused into the abdomen through a permanent catheter and allowed to dwell. Waste materials diffuse from the bloodstream across the peritoneum and equilibrate with the fluid before being removed. The exchanges are typically done multiple times a day, which can create inconvenience for the patient. Even so, outcomes for PD tend to be better than HD in the short term, and it’s also cheaper. Peritoneal catheters are usually surgically placed, though placement by IR is increasing in some practice settings. Aside from this, IR does not have a significant role in the care of these patients. With hemodialysis, the main challenge is establishing and maintaining vascular access. Dialysis machines require blood flow in a range somewhere between the rates seen in arteries and veins (> 300 mL/min). Another requirement is ease of access for the dialysis technicians to get the patient hooked up to the machine. Central venous access with a dialysis catheter is convenient in that it requires no needle puncture and achieves adequate flow. These lines are acceptable for short-term dialysis needs, but long-term use increases the risk of infection, making it unacceptable for chronic dialysis. The preferred route of long-term hemodialysis is an arteriovenous fistula (AVF) or graft (AVG). AV fistulas offer a permanent and easily accessible vascular access site for dialysis patients. Most fistulas are created by vascular surgery, with directly suturing of a vein to an artery. A percutaneous IR procedure for the endovascular creation of an AVF is being studied and may become an alternative to surgical creation in the near future. Preoperative imaging of the arm with duplex ultrasound or angiography is important to size the vein and to rule out any pre-existing arterial inflow or venous outflow obstruction that might compromise fistula viability. The preferred site for fistula creation is in the distal nondominant arm (radial–cephalic). If a distal site is not possible due to vessel occlusion or inadequate vessel size, fistulas can be created more proximally (brachiocephalic, transposition brachiobasilic), but these are less desirable (▸Fig. 10.1). Prior to being used for dialysis, an AV fistula needs time to mature. The newly created fistula dilates over time to the point of becoming superficial and easily palpable. With the increased blood flow, the vein also becomes “arterialized.” The walls thicken, allowing the vessel to better withstand the regular trauma that occurs with needle puncture. Bear in mind, a fistula will need to be punctured and accessed multiple times a week, for the rest of the patient’s life. An ideal fistula follows the “rule of sixes”: at 6 weeks, the fistula should be 6 mm in diameter, less than 6 mm deep, 6 cm in useable length, and have flow rates that exceed 600 mL/min (▸Table 10.1). In reality, time to full maturation can range between 2 and 4 months. Duplex fistula studies can be obtained to track progress. If a suitable vein is unavailable and an AV fistula cannot be created, an AV graft may be necessary. AV grafts rely on a prosthetic tube to bridge the artery and vein, requiring two anastomoses (▸Fig. 10.2). A common AV graft is the forearm loop AV graft. The arterial side of the graft is anastomosed to the brachial artery while the venous side of the graft is anastomosed to either the basilic, cephalic, or antecubital vein. AV grafts do not require time to mature and can be used immediately. In comparison to fistulas, grafts are more likely to become infected and typically have a shorter lifespan, so they should only be used if the anatomy is not suitable for an AVF. In general, the creation of an anastomosis between artery and vein introduces a host of hemodynamic and physiologic changes within the access site and its upstream and downstream vasculature. Increased flow into the access site and anastomosed venous segment causes an increase in wall shear stress, which in turn results in dilation of the vessels at the access site. Similarly, an increase in transmural pressure stimulates proliferation of smooth muscle, leading to thickening of the vessel wall. This dilation and wall thickening are the expected changes associated with appropriate fistula maturation.
At 6 weeks, a fistula should be… |
6 mm in diameter |
< 6 mm under the skin |
6 cm in useable length |
Flow rates > 600 mL/min |
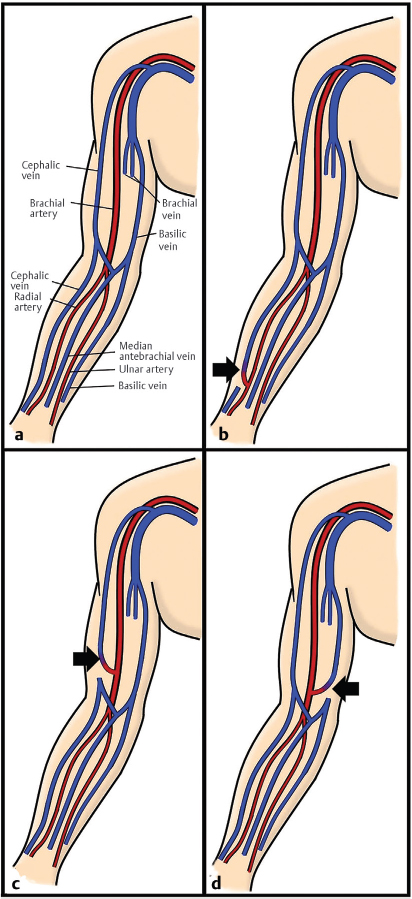
Fig. 10.1 Depiction of (a) normal upper extremity vascular anatomy. (b) A radiocephalic arteriovenous (AV) fistula. (c) A brachiocephalic AV fistula. (d) A brachiobasilic AV fistula. (These images are provided courtesy of Christopher Molloy, MD, Kaiser Permanente Los Angeles.)
10.1 Dialysis Access Dysfunction
As described, dialysis access creation leads to desirable vascular and physiologic changes necessary for fistula or graft use. However, changes in flow dynamics can also have deleterious consequences. For example, turbulence within the vessel as blood flows in and out of the dialysis access site exposes the vessel walls to an inconsistent pattern of wall shear stress. Areas of low wall shear stress induces a proinflammatory state and results in neointimal hyperplasia. This is one of the underlying causes of stenoses.
Fig. 10.2 Depiction of an arteriovenous graft between the brachial artery and the cephalic vein. (This image is provided courtesy of Christopher Molloy, MD, Kaiser Permanente Los Angeles.)
Early dialysis access dysfunction occurs anytime between fistula or graft creation and the first 3 months of use, most commonly as a result of fistula nonmaturation. A nonmatured fistula cannot be cannulated due to a combination of inadequate flow rate, suboptimal dilation of the arterialized vein, and/or a fistula depth too far below the skin.
In order for a fistula to be accessed and achieve flow rates necessary for the machine, the vessel needs to dilate. If the artery or vein chosen for the fistula are poor quality, the total dilation may be limited. Similarly, poor surgical technique may lead to incomplete dilation or a fistula that is too deep. Any additional insults on top of this, including the development of inflow or outflow stenoses, can also limit the ability of the fistula to dilate and reach a sufficient flow rate.
Grafts do not go through the process of maturation as fistulas do and can be used for dialysis relatively quickly (sometimes immediately). However, downstream neointimal hyperplasia can also occur shortly after placement due to the same physiologic changes.
Patients with matured fistulas can have varying degrees of dysfunction. Some may enjoy a relatively complication-free period, while others run into problems not long after the access is created. The dysfunction of matured fistulas and grafts can be due to stenoses anywhere along the arterial and venous limbs.
Arterial stenoses are more likely to be pre-existent rather than sequelae of access creation. The typical patient will have known peripheral arterial disease or risk factors for it (diabetes, smoking history, older age). Detection of arterial stenoses usually occurs with a nonmatured fistula and a low flow rate.
Venous stenoses are the most common reason for referral to IR. In addition to the instigating effect of turbulent flow, repetitive needle puncture of the access site causes endothelial cell damage and the release of proinflammatory cytokines, furthering the propensity for neointimal hyperplasia and stenosis in the downstream vein. Stenoses affecting the central veins can lead to venous hypertension as pooling increases hydrostatic pressure. This may result in extremity edema, prolonged bleeding time after venipuncture, and aneurysmal dilation of the access. Decreased flow rates will be noted during dialysis.
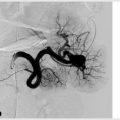
Repetitive needle punctures at the access site can weaken the vessel wall and form pseudoaneurysms (▸Fig. 10.3). Increased blood flow leads to vessel dilation and aneurysm formation proximal and distal to the access site. Pseudoaneurysms are unsightly but also present a risk of rupture.
The complications above are worrisome, but do not necessarily prevent the patient from getting dialysis. In contrast, dialysis can be completely interrupted when a venous stenosis precipitates thrombosis in the fistula or graft. Hypotension, either during the dialysis session or from another process, is a known risk factor for the development of thrombosis in the setting of an underlying venous stenosis. Excessive compression of the access site after dialysis, or simply from the patient sleeping on it wrong, can also contribute. Thrombosis is detected when there is a loss of thrill or bruit over the access site. The thrombus itself is unlikely to cause any damage, but the more pressing issue is the interruption of scheduled dialysis until access can be restored.
Unfortunately, stenosis and thrombosis are a part of the natural history of fistulas and grafts for many patients. Not uncommonly, patients with matured fistulas will enter a frustrating and chronic cycle of access complication, intervention, further complication, repeat intervention, and so on.
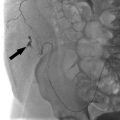
Another unique complication associated with AV fistulas/grafts is related to hemodynamic changes within the surrounding arm vasculature. As blood flows into the fistula through the feeding artery, perfusion to distal tissues (forearm, hand, etc.) diminishes initially while collateral flow is established. This in itself usually does not result in significant issues for the patient. Steal syndrome occurs when too much arterial flow is directed into the fistula, in effect “stealing” blood from the distal extremity (▸Fig. 10.4). This can result in episodic or constant ischemic pain, particularly when the arm is being used. It is more commonly seen in those with underlying atherosclerotic disease, however, it may affect those with clean arteries in the presence of a particularly high-flow fistula.