and John P. Deveikis2
(1)
Department of Surgery, Division of Neurosurgery, and Departments of Radiology and Neurology, University of Alabama, Birmingham, AL, USA
(2)
Bayfront Medical Center, St. Petersburg, FL, USA
Abstract
Arteriovenous malformations (AVMs) are congenital vascular lesions that may appear throughout the central nervous system. They consist of direct connections between arteries and veins, without an intervening capillary bed. They are believed to be about one-tenth as common as intracranial aneurysms. Vein of Galen malformations are a separate entity and are discussed in the Appendix to this chapter.
Arteriovenous malformations (AVMs) are congenital vascular lesions that may appear throughout the central nervous system. They consist of direct connections between arteries and veins, without an intervening capillary bed. They about one-tenth as common as intracranial aneurysms. Spinal AVMs are discussed in Chap. 20. Vein of Galen malformations are a separate entity and are discussed in the Appendix to this chapter.
14.1 Pathophysiology
14.1.1 Pathology
1.
Gross appearance
(a)
Intracranial AVMs often resemble a ball of red and blue noodles, described by Cushing and Bailey as a snarl of tangled vessels.1
(b)
AVMs are frequently pyramidal-shaped lesions, with the base at and parallel to the cortical surface and the apex directed toward the ventricle.
(c)
The nidus may be compact or diffuse, and range in size from several millimeters to an entire hemisphere.
(d)
The adjacent brain parenchyma may be haemosiderin-stained from previous haemorrhage, and the overlying meninges may be thickened and fibrotic.2 Extensive gliosis, fibrosis, and calcification may be present.
2.
Histopathological features
(a)
Arteries
AVM arteries are abnormally dilated, with marked thinning in some regions and degeneration or absence of the media and elastic lamina.3 Degenerative changes are present, presumably due to wall shear stress caused by high flow.2 These include irregular thickening of the vessel wall in some regions, endothelial proliferation, medial hypertrophy, and multilaminated, thickened basal laminae.4
(b)
Nidus
Nidal vessels may contain a hypertrophic media, blurring the distinction between arteries and veins.
Aneurysms and islands of sclerotic tissue may be present within the nidus.
(c)
Veins
“Arterialized” veins may exhibit thickening of the vein wall due to cellular proliferation.2
Although thickened AVM veins may grossly resemble arteries, they lack an organized elastic lamina and therefore are not truly arterial structures.
(d)
Functional brain tissue is usually not present within an AVM, although in diffuse lesions, AVM vessels may be separated by normal tissue.
14.1.2 Etiology
1.
AVMs are assumed to appear during fetal development between the 4th and 8th weeks of life.
2.
The precise etiology of AVMs is unclear. Some theories:
(a)
AVMs represent persistent direct connections between arteries and veins within the primitive vascular plexus.
(b)
AVMs are dynamic and result from a derangement in vessel growth, i.e., a “proliferative capillaropathy”.7
(c)
AVMs result from a dysfunction of the remodeling process at the junction between capillaries and veins.8
14.1.3 Physiology
1.
Haemodynamic characteristics
(b)
Steal phenomenon. In some cases, high-flow diversion of blood flow through an unruptured AVM is believed to produce symptoms by reducing perfusion in adjacent normal brain tissue.11 Steal, as a mechanism of symptoms, is thought to be very rare or nonexistent.
Regional CBF surrounding the lesion may be reduced.12
Adaptive changes in autoregulation in peri-lesional brain tissue may occur.13
Evidence against steal phenomenon: In a prospective study of 152 patients with unruptured AVMs, only 2 (1.3%) had focal neurological symptoms possibly attributable to steal phenomenon.14 TCD examinations showed no relation between feeding artery pressure or flow velocities and neurological deficits; the authors took these findings as evidence against steal as a pathophysiological mechanism in most patients.
14.2 Clinical Features
14.2.1 Epidemiology
1.
Prevalence
(a)
(b)
In contrast to unruptured intracranial aneurysms, which have been diagnosed at an increasing rate in recent years, there has been a decline in the rate of detection of brain AVMs in recent years.23
2.
14.2.2 Anatomic Features
1.
Location
(b)
(c)
Eloquent tissue (sensorimotor, language, or visual cortex; hypothalamus or thalamus; internal capsule; brainstem; cerebellar peduncles, or cerebellum) is involved in up to 71% of the cases.31
2.
Feeding vessels
a)
Feeding arteries have been divided into three types:32
Terminal: Arteries which may supply normal tissue proximally but terminate within the AVM.
Pseudo-terminal: Feeders that supply normal brain distal to their supply to the AVM.
Indirect (aka en passage): feeders that typically arise at right angles from larger normal arteries.
14.2.3 Conditions Associated with AVMs
14.2.3.1 Familial Intracranial AVMs
Most intracranial AVMs are sporadic. “Familial” AVMs are rare; only 53 cases in 25 families have been reported.35 A systematic review of reported cases found:35
1.
Mean age at diagnosis: 27 years, which was younger than in patients with sporadic AVMs.
2.
Patients with familial AVMs did not differ from the reference populations with respect to sex and mode of presentation.
3.
In families with familial AVMs in successive generations, the age of the child at diagnosis was younger than the age of the parent at diagnosis, which suggests clinical anticipation.
14.2.3.2 Hereditary Haemorrhagic Telangiectasia
Hereditary haemorrhagic telangiectasia (aka Rendu-Osler-Weber syndrome) is group of autosomal dominant disorders of vascular structure, affecting the brain as well as the nose, skin, lungs, and gastrointestinal tract. Similar cases were independently reported by Rendu,36, Osler,37, and Weber38 around the beginning of the last century.
1.
Diagnosis is based on four primary clinical features:39
(a)
Spontaneous recurrent nosebleeds
(b)
Muco-cutaneous telangiectasia
(c)
Visceral involvement
(d)
An affected first-degree relative
Definite: three criteria are present
Suspected: two criteria are present
Unlikely: one criterion is present
2.
3.
Clinical features
a)
Central nervous system
Cerebrovascular abnormalities associated with Rendu-Osler-Weber syndrome include AVMs, telangiectasias, cavernous malformations, and aneurysms.44,45
An MRI screening study found a prevalence of cerebrovascular lesions of 23%46
AVMs are the most common vascular lesions. Intracranial or spinal AVMs are present in some 10–15% of patients.43,47–49
The incidence of haemorrhage in patients with an AVM and Rendu-Osler-Weber syndrome is comparable to (or less than) the incidence in the non-Rendu-Osler-Weber population, although haemorrhage is six times more common among women.48,49
Cerebral ischaemic events and brain abscesses may be attributable to right-to-left shunting due to pulmonary AVMs.50
(b)
Epistaxis
Spontaneous epistaxis from telangiectasias of the nasal mucosa is the most common clinical manifestation; in 80% of cases epistaxis is the first clinical symptom of the disease.52
More than 50% of patients have recurrent epistaxis before the age of 20.53
Epistaxis occurs in a biphasic 24-h pattern, with a primary peak in the morning and a smaller second peak in the evening.54
(d)
4.
Pathophysiology
a)
The initial morphologic change of hereditary haemorrhagic telangiectasia consists of focal dilatations of postcapillary venules accompanied by a diminishing capillary network.43,58 As the venules enlarge over time, they become tortuous and connected to enlarging arterioles, eventually forming direct arteriovenous fistulae.
14.2.3.3 Wyburn-Mason Syndrome
Wyburn-Mason syndrome (aka unilateral retinocephalic vascular malformation or Bonnet-Dechaume-Blanc syndrome) is a rare condition characterized by the presence of AVMs in the brain and retina. Roger Wyburn-Mason published the first English language analysis of this syndrome in London in 1943.63 Théron and colleagues published an analysis of 25 cases.64
1.
Wyburn-Mason syndrome is congenital, nonhereditary, and without sex or race predilection.
2.
The initial diagnosis of the syndrome is usually made when a retinal AVM is detected.
3.
The associated intracranial vascular lesion is unilateral, related to the optic pathway, and frequently involves the optic nerve, chiasm, optic tract, and basal ganglia.64 In some cases the AVM may extend to the occipital lobe.
14.2.3.4 Sturge-Weber Syndrome
Sturge-Weber syndrome (aka encephalotrigeminal angiomatosis) is a neurocutaneous disorder. Patients typically present with angiomas involving the leptomeninges, retina, and the dermatomes (port wine stains) of the face. The leptomeningeal venous angioma consists of numerous small tortuous, thin-walled vessels lying in the pia and adjacent hemisphere, typically in the posterior parietal and anterior occipital lobes. Associated intracranial AVMs have been reported.65,66
14.2.3.5 Moyamoya Syndrome Associated with AVM
Numerous case reports show that AVMs may be seen in patients with the occlusive changes in moyamoya.67–69 The chronic ischaemia in moyamoya syndrome results in an environment of high levels of angiogenic factors to improve development of collaterals (see Chap 19). Interestingly, there have been reports of de novo appearance of AVMs in pediatric patients being followed with moyamoya syndrome.70,71 The process of angiogenesis in the development of AVMs may be similar to the angiogenesis that produces the collateral vessels in moyamoya.72 On the other hand, there has also been a report of de novo development of the bilateral stenotic changes of moyamoya 9 months after radiosurgery for a right temporal AVM.73
14.2.4 Natural History
Natural history data comes from numerous retrospective studies and several prospective studies. Estimates of the annual rate of haemorrhage from an AVM range from <2% to 17.8%.28,74–79 The risk of haemorrhage from an AVM depends strongly on whether there has been a previous haemorrhage.
1.
3.
Spontaneous regression
(a)
Rare. In a review of 700 cases, a total of six cases (0.9%) of angiographically documented lesions disappeared on follow-up angiograms.86 Three of these cases occurred in patients that had undergone partial resection of the lesion.
14.2.4.1 Lifetime Risk of Haemorrhage
Assuming a constant annual risk of haemorrhage of 2–4%, the lifetime risk of haemorrhage can be estimated by the following formula:87
Lifetime risk = 1 − (Risk of no haemorrhage)Years remaining of life
Alternative method. Assuming a 3% annual risk, lifetime risk can be approximated as follows:88
Lifetime risk (%) = 105 − The patient’s age in years (Table 14.1).
Table 14.1
Life expectancy table and projected lifetime risk of AVM haemorrhage
Age interval | Average no. of years remaininga | Estimated lifetime risk of rupture according to annual risk of ruptureb | ||
|---|---|---|---|---|
2% | 3% | 4% | ||
5–9 | 72.9 | 0.77 | 0.89 | 0.95 |
10–14 | 67.9 | 0.75 | 0.87 | 0.94 |
15–19 | 63.0 | 0.72 | 0.85 | 0.92 |
20–24 | 58.2 | 0.69 | 0.83 | 0.91 |
25–29 | 53.5 | 0.66 | 0.8 | 0.89 |
30–34 | 48.7 | 0.63 | 0.77 | 0.86 |
35–39 | 44 | 0.59 | 0.74 | 0.83 |
40–44 | 39.3 | 0.55 | 0.7 | 0.8 |
45–49 | 34.8 | 0.5 | 0.65 | 0.76 |
50–54 | 30.3 | 0.46 | 0.6 | 0.71 |
55–59 | 26.1 | 0.41 | 0.55 | 0.66 |
60–64 | 22 | 0.36 | 0.49 | 0.59 |
65–69 | 18.2 | 0.31 | 0.43 | 0.52 |
70–74 | 14.7 | 0.26 | 0.36 | 0.45 |
75–79 | 11.5 | 0.21 | 0.3 | 0.37 |
80–84 | 8.8 | 0.16 | 0.24 | 0.3 |
85–89 | 6.5 | 0.12 | 0.18 | 0.23 |
90–94 | 4.8 | 0.09 | 0.14 | 0.18 |
95–99 | 3.6 | 0.07 | 0.1 | 0.14 |
14.2.4.2 Risk Factors for Haemorrhage
Risk of haemorrhage is not uniform across the population of patients with AVM,89 and appears to vary widely according to a number of patient characteristics. However, data about risk factors for haemorrhage must be interpreted with caution. For nearly every factor that has been associated with AVM haemorrhage, there is at least one other study that has not found a significant association. 90
2.
AVM size – controversial
(b)
16.
A polymorphism in the inflammatory cytokine IL6 (i.e., patients homozygous for the interleukin (IL)-6-174 G allele).106
14.2.5 Presentation
1.
Haemorrhage
(a)
Most common symptom at presentation, occurring in some 53% of patients at initial diagnosis.26
2.
Seizures
(a)
(c)
(d)
Seizures associated with parietal lobe AVMs are typically focal, whereas seizures due to frontal lobe AVMs are frequently generalized.108
3.
4.
Developmental learning disorders
(a)
Patients with AVMs are more likely to have developmental learning disorders than patients with other intracranial disorders, even many years prior to the diagnosis of the AVM.115 This may be due to subtle injury to the brain by the AVM, displacement of functional tissue by the AVM, or steal phenomenon.
14.2.6 Special Section: Cerebral Proliferative Angiopathy
This important clinical entity (aka diffuse cerebral angiomatosis116 or diffuse AVM)117 was termed cerebral proliferative angiopathy (CPA) by Lasjaunias and colleagues in Stroke in 2008.118 CPA (Fig. 14.2) is a vascular malformation that can be confused with a brain AVM, but has several important features that distinguish it from classic brain AVMs. These lesions comprise some 3.4% of brain AVMs.118 The existing literature on the topic consists mostly of Lasjaunias’ series of 49 patients and several case reports.119–121
1)
Features that distinguish cerebral proliferative angiopathy from brain AVMs:118
a)
Large size (usually lobar or hemispheric)
b)
Absence of dominant feeders or flow-related aneurysms
c)
Presence of transdural supply (which is unusual in classic brain AVMs)
d)
Presence of proximal stenosis on feeding arteries
e)
Absence of large, early-draining veins
f)
Normal brain tissue is intermingled between the vessels
2)
Epidemiology and presentation
a)
Some 67% of patients are female (compared to 48% of patients with brain AVMs)
b)
Mean age at diagnosis: 22 years
c)
Presenting symptom:
i)
Seizures (45%)
ii)
Severe headaches (41%)
iii)
Haemorrhage (12%)
iv)
Stroke, TIA or other neurological deficit (16%)
3)
Pathophysiology
a)
Unlike brain AVMs, CPA vessels appear to be proliferative, which may be a response to cerebral ischaemia. Angiogenesis and neovascularization is evidenced by meningeal arterial involvement,122 which is not typical of brain AVMs. Perfusion-weighted imaging has demonstrated evidence of cortical ischaemia,123 and stenosis of feeding vessels is present in 39% of cases.118 Taken together, the evidence suggests that cerebral ischaemia may drive the formation of CPA lesions.
4)
Imaging
a)
CPA lesions tend to be large and diffuse, with normal brain parenchyma intermingled with the vascular elements.
b)
Catheter angiography should include imaging of the external carotid arteries because of the frequent presence of meningeal feeders.
c)
Draining veins are not conspicuous and early draining veins are usually not present.
5)
Management
a)
Treatment with surgery, radiosurgery, and embolization is problematic because of the large size of CPA lesions and the presence of normal brain tissue among the vessels.
i)
Selective embolization of “fragile areas” in patients presenting with haemorrhage has been advocated.124
b)
Burr hole encephalodurosynangiosis procedures provided improvement in headaches in two patients in Lasjaunias’ series.118
c)
For patients presenting with seizures without haemorrhage, treatment limited to medical anticonvulsant therapy may be the most prudent option.
14.2.7 Imaging
1.
CT/CTA
(a)
CT is still the best imaging technique to check for an acute haemorrhage.
(b)
A noncontrast CT of an unruptured AVM may appear normal; sensitivity can be increased by using IV contrast or doing a CTA.
CT findings with AVMs:
Heightened vascularity.
Serpentine, enlarged veins.
In some cases, perilesional atrophy and/or hydrocephalus.
(c)
CTA: AVMs are typically best seen on MIP images.
2.
3.
Angiography
(a)
Catheter angiography provides information about AVMs that is superior to complimentary compared to other imaging techniques. Advantages include:
Greater sensitivity.
Able to clarify anatomy of feeding vessels and draining veins (e.g., distinguishes between ACA and MCA contributions to a cerebral convexity lesion).
Best imaging technique to identify intranidal aneurysms.
Able to determine arteriovenous transit times.
b)
Angiography remains the gold standard for the evaluation of AVMs, and the authors of this handbook believe that it should be considered for every patient with an intracranial AVM, or an intracerebral haemorrhage that may be due to an AVM.
Yield of angiography in patients with spontaneous ICH (i.e., the chance of finding an underlying vascular abnormality:127
Patients age ≤45 years: 50%
Patients age >45 years: 18%
Patients without a history of hypertension: 44%
Patients with a history of hypertension: 9%
(c)
Complications: A systematic review found that the risk of complications of angiography in patients with AVMs (0.3–0.8%) is significantly lower than for patients being evaluated for TIA or stroke (3.0–3.7%).128
14.2.8 Special Section: AVM mimics
A number of cerebrovascular lesions can masquerade as brain AVMs on imaging (Figs. 14.1, 14.2, 14.3, and 14.4).
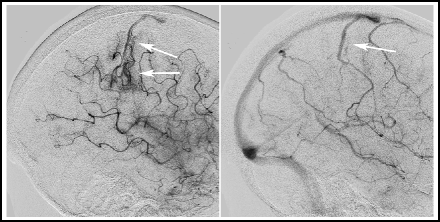
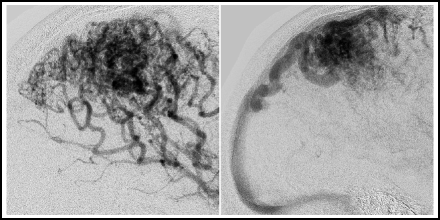
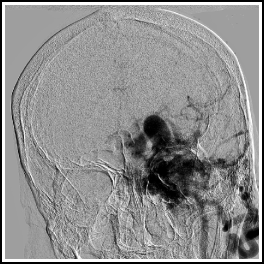
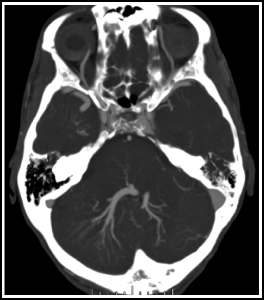

Fig. 14.1
Ischaemic stroke with arteriovenous shunting. This patient had an embolic stroke. Several days after the event, the arterial phase angiogram shows heightened vascularity and an early draining vein in the affected territory (arrows, right). The venous phase angiogram shows the early-appearing vein to be a normal cortical vein (arrow, left).

Fig. 14.2
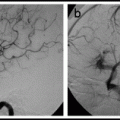
Cerebral proliferative angiopathy.118 Seventeen year old boy who presented with a seizure. Note the typical features of cerebral proliferative angiopathy that distinguish it from an AVM, such as large, diffuse nidus and absence of early draining veins. Lateral view, right parietal area, arterial phase (left) and venous phase (right).

Fig. 14.3
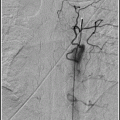
Intracranial dural arteriovenous fistula. This patient, with a large Borden 3 left transverse/sigmoid sinus dAVF, was originally misdiagnosed as having a brain AVM. Frontal view with injection into the left CCA.

Fig. 14.4
Large developmental venous anomaly. This patient, with a large posterior fossa DVA, was originally misdiagnosed as having a brain AVM. Axial MIP image from a CTA.
14.3 Management
Management options for patients with an AVM are
1.
Expectant management
2.
Surgery
3.
Radiosurgery
4.
Embolization
5.
A combination of embolization, radiosurgery, and/or surgery
Obviously, AVMs and patients with them vary greatly, and so the management strategy for any given patient must be highly individualized. Mainstream thinking presently favours surgery or radiosurgery for most patients;129 embolization is usually most useful as a preparatory step prior to surgery or radiosurgery. Conservative management is gaining favour for large or difficult-to-treat lesions, and for patients at high risk of complications.130
14.3.1 Expectant Management
Non-surgical and non-interventional management of some patients with AVMs is appropriate. Some authors, considering the natural history of asymptomatic AVMs and the relative low morbidity associated with haemorrhage of some lesions, argue against the routine treatment of asymptomatic lesions.131 An ongoing NIH-sponsored multicenter study, A Randomized Trial of Unruptured Brain Arteriovenous Malformations (ARUBA), is comparing treatment of unruptured lesions with conservative management (http://www.arubastudy.org)132 Large AVMs may also warrant conservative management, considering the difficulty and morbidity of treatment.
14.3.2 Surgery
Surgical resection of brain AVMs is the “gold standard” for the treatment of small, accessible lesions.129,133,134 Decision making in most cases begins with stratification according to the Spetzler–Martin grading system (Table 14.2), which is currently the most commonly used system. A decision analysis model suggested that surgical resection of small, asymptomatic AVMs, assuming a risk of major neurological morbidity and mortality <6.8%, offers the greatest overall quality of life over time compared to observation or radiosurgery.135
Table 14.2
Spetzler–Martin scale
Lesion characteristic | Number of points | |
|---|---|---|
Size | Small (<3 cm) | 1 |
Medium (3–6 cm) | 2 | |
Large (>6 cm) | 3 | |
Location | Non-eloquent site | 0 |
Eloquent site (sensorimotor, language, or visual cortex; hypothalamus or thalamus; internal capsule; brainstem; cerebellar peduncles, or cerebellum) | 1 | |
Pattern of venous drainage | Superficial only | 0 |
Any deep | 1 |
14.3.2.1 Surgical Outcomes
1.
Obliteration rates.
(a)
(b)
Spetzler–Martin grades IV–V: There is a paucity of data on angiographic obliteration rates after surgery for high grade AVMs, partly because multimodality treatment strategies are frequently used. Separate discussions of Large AVMs and Multimodality Treatment appear below.
(c)
For a discussion of surgery compared to radiosurgery, see below.
2.
Complications. A systematic review of 25 reports, including 2,425 patients, found an overall rate of post-operative mortality of 3.3% and permanent morbidity of 8.6%.142
14.3.3 Surgery: Practical Issues
1.
Timing of surgery. AVMs do not carry the same high rehaemorrhage risk that ruptured aneurysms do; therefore, timing of surgery depends on several factors other than rehaemorrhage risk. Most authors recommend that surgery be done on an elective basis,129,148,149 days to weeks after the ictus, to allow the patient to recover from the initial event and to allow the clot to liquefy. Others advocate earlier surgery in most cases.150
(a)
Early surgery is indicated when
The clot has significant mass effect and the patient will benefit from evacuation of the hematoma.
The lesion is surgically accessible.
(b)
Late surgery (several weeks or more after the haemorrhage) is indicated when:
The clot burden is relatively low.
The patient is relatively poor surgical candidate soon after the initial haemorrhage.
Imaging studies do not show the AVM clearly, and a delayed, detailed angiogram may show the lesion more clearly.
2.
Surgery combined with embolization or radiosurgery. See the sections below on Radiosurgery and Embolization.
3.
Intraoperative and postoperative angiography: Either intraoperative or postoperative angiography is always indicated, to ensure complete obliteration of the lesion.
(a)
Intraoperative angiography
Advantages: Allows for detection, and removal, of residual AVM during the operation.
Disadvantages: Adds time to the operation; angiography in the OR is usually lower-quality than biplane angiography in a dedicated neuro-angio suite.
Results: A review of published reports of intraoperative angiography found that the results of the intraoperative angiogram altered the management of the case in an average of 15% of the time (range: 5.6–57%).151
(b)
Post-op angiography
Results: In a series of 324 patients undergoing post-op angiography craniotomy for AVM resection, 1.8% were found to have residual lesions.152
4.
Surgical complications
(a)
(b)
Cerebral oedema
Perioperative cerebral oedema occurs in ≤3% of cases136,155–158 and may first occur in the operating room or up to 11 days after surgery.155 Cerebral oedema may occur after AVM surgery or embolization.159
Post-treatment cerebral oedema is believed to be attributable in most cases to (1) normal perfusion pressure breakthrough or (2) occlusive hyperaemia.
Normal perfusion pressure breakthrough. 160 This theory maintains that the tissue around an AVM is subject to chronic steal because of diversion of flow into the AVM, resulting in sustained dilation and loss of autoregulation. Presumably, in some cases the vessels in this tissue are unable to autoregulate when normal perfusion is reestablished after resection of the AVM, resulting in oedema and haemorrhage.
Management
Standard measures to control cerebral oedema in this setting include:161
(a)
Head CT to exclude haemorrhage (obviously).
(b)
The head and neck should be maintained in a neutral position to minimize jugular vein compression and obstruction of CSF outflow.
(c)
IV mannitol.
(d)
Ventriculostomy.
(e)
(f)
Decompressive craniectomy (or removal of the bone flap).
(g)
High-dose barbiturate anesthesia.
(c)
Rehaemorrhage
Overall incidence of early rehaemorrhage after surgery (≤1 week) is 2%.164 Risk factors for rehaemorrhage include higher grade AVMs and the presence of lenticulostriate feeders.164
Often attributable to residual AVM.
Aggressive post-operative blood pressure control can minimize risk of rehaemorrhage.
(d)
Vasospasm
Symptomatic vasospasm, attributable to extensive dissection and exposure of major intracranial arteries, occurs in <1% of cases.7
14.3.4 Radiosurgery
Radiosurgery involves the administration of multiple beams of radiation; each beam is delivered from a different direction, and all beams converge on the target, or isocenter. The radiation dose in the isocenter is high but the radiation dose received by non-targeted structures is relatively low. Advantages of radiosurgery are that it is minimally invasive, relatively low-risk, and useful for treatment of surgically inaccessible lesions. Disadvantages include a latency period (usually 2–3 years) until AVM obliteration occurs, and that it is most effective for smaller lesions.
14.3.4.1 Radiosurgery Techniques
1.
Gamma knife.
(a)
Most widely used platform for AVM radiosurgery.
(b)
Two hundred and one gamma ray beams from cobalt-60 sources pass through holes (collimators) in a helmet and converge on the isocenter. Dosing is controlled by determining the size of the collimators and the exposure time.167
2.
Linear accelerator (LINAC).
(a)
LINACs use microwaves to accelerate electrons that then collide with a target to generate high energy photons. The LINAC is mounted on a gantry that rotates through an arc and concentrates radiation energy on the isocenter. Dosing is controlled by using multiple intersecting arcs and beam weight adjustment. Current LINAC devices include Novalis® (BrainLAB, Helmstetten, Germany), X-Knife™ (Radionics, Burlington MA), Trilogy™ (Varian Medical Systems, Palo Alto, CA), and CyberKnife® (Accuray, Sunnyvale, CA).
3.
Particle beam.
(a)
Charged particles are delivered rather than photons. The theoretical advantage of particle beam treatment over photon beam radiosurgery is that the energy deposition is more concentrated (and tissue exposure outside of the target is lessened) due to the Bragg peak effect. Another theoretical advantage is that the relative biological effectiveness is higher compared to other techniques. Both protons and helium nuclei have been used to treat AVMs.168,169 Disadvantage is that particle beam radiosurgery requires a relatively expensive cyclotron or synchrotron.
14.3.4.2 Mechanism of AVM Obliteration in Radiosurgery
The earliest and primary effect of radiosurgery is damage to AVM endothelial cells. Progressive occlusion of the vessel lumen occurs during a sequence of events that is similar to wound healing.170 Endothelial damage induces the proliferation of smooth muscle cells and myofibroblasts and an accumulation of extracellular collagen, causing stenosis and occlusion of the nidus.170,171 A chronic inflammatory response also contributes to the formation of granulation tissue in the region of the AVM.
14.3.4.3 Radiosurgery Outcomes
1.
Obliteration rates. Rates of angiographic cure depend primarily on lesion size. Most of the following results are at 2–5 year follow-up.
(c)
Brainstem AVMs. These lesions represent a special case because of the high morbidity of rupture and because of the need for excellent three-dimension dose conformality, to minimize injury to adjacent vital structures. In a recent series of 44 patients with brainstem AVMs treated with radiosurgery, the obliteration rate was 52% at 5 years; disease-specific survival was 86% at 10 years after treatment.178
2.
Effect on haemorrhage risk
(a)
Risk of haemorrhage persists during the latency period between radiosurgery and AVM obliteration, but may be reduced compared to the risk of haemorrhage prior to radiosurgery.179 One study found the annual risk of haemorrhage during the 2-year latency period to be 4.8%;180 more recent studies found annual risk of haemorrhage after radiosurgery to be 1.8–2.8%.83,181
(b)
The presence of an unsecured proximal aneurysm is associated with an increased risk of haemorrhage during the latency period.180
(c)
Even with complete angiographic obliteration, the risk of haemorrhage may not be zero; Shin and colleagues estimated the risk of rebleeding after complete nidus obliteration to be 0.3% per year.182
3.
4.
Complications. Pooled data from 1,255 patients:187
(a)
Overall rate of neurological complications (transient or permanent neurologic deficits): 8%
(b)
Permanent neurologic deficits: 4.8%
(c)
Complications:
Radiation injury to brain parenchyma (6.4%)
Cranial nerve injury (1%)
New or worsened seizures (0.8%)
Death (0.2%)
14.3.4.4 Radiosurgery: Practical Issues
1)
Dosing and treatment strategy
a)
Dose
i)
The applied radiation dose is inversely proportional to the irradiated volume. To determine the dose of radiation, a dose/volume curve is used.
ii)
Dose–response studies have found meaningful responses up to 25 Gy.188 Above this point, there is minimal incremental increase in obliteration rates but a significant increase in complications.
iii)
b)
Fractionation
i)
Fractionation (e.g., two or more separate target volumes are planned, with the treatments separated in time) has been used for large AVMs.
(1)
A hypofractionation scheme (dose of 25–30 Gy in 5–6 daily fractions) used for large AVMs (>5 cm) resulted in a reduction of AVM volume by 44% per year.193
2)
Complication management
a)
Transient brain oedema is a source of headaches and neurologic deficits in some patients after radiosurgery. Some operators use low-dose oral dexamethasone therapy for 2 weeks after treatment.174 Delayed symptoms attributable to brain oedema usually respond to a short course (2–3 days) of IV dexamethasone.
3)
Follow-up imaging
a)
Follow-up catheter angiography is necessary in all patients 2–3 years after radiosurgery for an AVM, because of the significant (5–25%) chance of residual and because complete lesion obliteration is necessary to minimize haemorrhage risk.194
14.3.5 Surgery Compared to Radiosurgery
Several reports have compared surgery to radiosurgery, with the expected results. Surgery is associated with a higher cure rate with a lower rehaemorrhage risk, while radiosurgery is less morbid.
1.
Pikus and colleagues compared a surgical series to published radiosurgery reports and found advantages in surgery in terms of angiographic cure rates and rehaemorrhage risk, although surgery had a significantly higher rate of neurological complications.139
2.
Nataf and coworkers compared two series of patients with lesions that were considered equally suitable for treatment by surgery or radiosurgery.198 Although the rate of cure was similar for both groups of patients, neurological morbidity was higher after surgery and recurrent bleeding was more frequent after radiosurgery.
14.3.6 Embolization
14.3.6.1 A Brief History of Embolization of Intracranial AVMs
The first report of embolization of a brain AVM appeared in 1960.199 A patient with a large left Sylvian fissure AVM underwent surgical exposure of the cervical carotid artery, and four spheres of methyl methacrylate, measuring between 2.5 and 4.2 mm in diameter, were embolized into the lesion. Angiography showed near total occlusion of the lesion and good filling of normal vessels. This technique evolved into a strictly endovascular process with placement of a large-bore catheter in the femoral artery.200 The spheres were then injected one-by-one through the catheter into the cerebral circulation. The appearance of the spheres traveling through the vessels on fluoroscopy was strangely fascinating, and can be compared to watching a pinball at an arcade. An element of chance was always involved, and one could never be certain where they would land. Still, because of preferential flow to the AVM, most would end up in the feeding arteries to the lesion.
Limited further progress was made with AVM embolization during the 1960s, largely because of the absence of useful catheters and embolic agents. Flow-directed particulate embolization,201 balloon embolization,202 and delivery of embolic material through a punctured microballoon (the “calibrated leak balloon” technique)203,204 were reported in the 1970s.
An array of embolic agents were used in the 1970s and early 1980s, including silk threads, alcohol, polyvinyl alcohol (PVA) and isobutyl-2-cyanoacrylate (i-BCA). Reports of toxicity and carcinogenicity with i-BCA in animal studies205,206 led to the withdrawal of this material from the market and the introduction of N-butyl-2-cyanoacrylate (n-BCA).207
Advancements in catheter design in the 1980s permitted selective catheterization of AVM pedicles. The introduction of the flow-directed Magic catheter was a major breakthrough.208 With the additional availability of 0.010-in. wires and the addition of hydrophilic coating to the catheters, intranidal catheterization was possible.209
During the 1990s, n-BCA and polyvinyl alcohol emerged as the most popular embolization materials for AVMs. FDA approval of n-BCA (Trufill, Codman Neurovascular, Raynham, MA), was granted in 2000. While the principal advantage of n-BCA is its resistance to recanalization, compared to PVA (which is prone to recanalization),210 the adhesive properties of n-BCA require great care during injection to minimize the risk of catheter retention. A randomized trial comparing n-BCA to PVA for preoperative embolization of AVMS found no difference and also that the two agents were equivalent in terms of percentage of nidus reduction and number of feeding pedicles embolized.211 Interestingly, although the overall rates of procedural complications were similar in both groups, patients treated with PVA had a significantly higher rate of post-resection haemorrhage compared to patients treated with n-BCA (17.8% vs. 4.8%).
Ethylene vinyl alcohol copolymer in dimethyl sulfoxide solution (Onyx, Micro Therapeutics, Inc., Irvine, CA) was introduced in 1990. Although Onyx was originally conceived of as an embolic agent for intracranial aneurysms, somewhat disappointing results in a North American randomized trial of the material for aneurysms212 were followed by encouraging initial results with the use of the agent for the treatment of AVMs.213 Onyx received FDA approval for presurgical embolization of AVMs in 2005.
14.3.6.2 Embolization Results
AVM embolization techniques and strategies are discussed in detail in Chap. 7, Intracranial Embolization.
1)
2)
3)
Embolization combined with surgery or radiosurgery (or both). Numerous reports of presurgical31,136,214,215,222–227 and pre-radiosurgical210,217,228–230 embolization have been published.
a)
Pre-surgery embolization.
i)
The goals of pre-surgery embolization are to reduce the volume of the nidus and to occlude deep and difficult-to-reach feeders when possible.
ii)
iii)
In a series comparing AVM surgery patients with presurgical embolization to those without it, complication rates and the proportion of patients with good or excellent outcomes were similar in both groups, despite the fact that the embolization group had higher grade lesions.222
iv)
Vinuela and colleagues suggested that occlusion of >75% of the AVM nidus facilitates surgical resection, while elimination of <50% does little to facilitate surgery.232
b)
Pre-radiosurgery embolization
i)
Controversial! Embolization prior to radiosurgery may reduce the effectiveness of radiosurgery
ii)
The aim of pre-radiosurgery embolization is to reduce the volume of the nidus.
iii)
Older reports indicated improved rates of AVM obliteration have been found when the volume is reduced with embolization to <10 cm.3,210,217 Volume – diameter relationships are listed in Table 14.3.
Table 14.3
Estimation of AVM volume based on diametera
Diameter (cm) | Volume (cm3) |
|---|---|
1 | 0.51 |
2 | 4.19 |
3 | 14.14 |
4 | 33.51 |
5 | 65.45 |
6 | 113.09 |
14.3.7 Specific Considerations
14.3.7.1 Associated Aneurysms
Most reports indicate that intracranial aneurysms are found in some 15–25% of patients with AVMs.242,243 One study of superselective angiography reported an incidence of associated aneurysms of 58%.103
1.
Classification and incidence of AVM-associated aneurysms
(a)
(b)
Get Clinical Tree app for offline access

Intranidal aneurysms
True intranidal aneurysms
True aneurysms in the AVM nidus can be distinguished from venous varices by their early appearance during the arterial phase on angiography.244

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree