Information obtained from brain imaging is now summarized in the form of various neuroimaging scores to help physicians in making therapeutic decisions and determining prognosis. The Alberta Stroke Program Early CT Score (ASPECTS) was devised to quantify the extent of early ischemic changes in the middle cerebral artery territory on noncontrast computed tomography. With its systematic approach, the score is simple, reliable, and a strong predictor of functional outcome. This review summarizes ASPECTS and other neuroimaging scores developed for risk prognostication and risk stratification with treatment in patients with acute ischemic stroke.
Brain imaging has revolutionized the treatment of patients with acute ischemic stroke. With the visual differentiation of hemorrhagic from ischemic stroke, thrombolytic therapy became feasible. The effort since then has been to use imaging techniques to help determine which patients would benefit most with thrombolytic therapy. With the availability of endovascular techniques capable of opening an occluded artery faster than intravenous (IV) recombinant tissue plasminogen activator (rtPA), imaging techniques have also been used to tailor therapy appropriate to a patient. This review summarizes current knowledge on the Alberta Stroke Program Early CT Score (ASPECTS) and other neuroimaging scores that help a clinician determine prognosis and decide on appropriate therapy in patients presenting with acute ischemic strokes.
ASPECTS
In the National Institute of Neurological Disorders and Stroke (NINDS) rtPA Stroke Study, computed tomography (CT) was used as a screening tool to exclude intracranial hemorrhage (ICH) before rtPA administration. The extent of early ischemic changes (EIC) on the baseline CT scan did not influence patient eligibility. Initial EIC definition was based on edema and mass effect. A total of 5.2% patients had evidence of such findings. Their presence was associated with a higher risk of symptomatic intracranial hemorrhage (sICH); however, no treatment-modifying effect was demonstrated. A more detailed re-review of the NINDS rtPA Stroke Study scans resulted in a higher prevalence of EIC (31%), largely due to a differing appreciation and definition of EIC. However, again no EIC-by-treatment interaction was statistically demonstrated.
The European Cooperative Acute Stroke Study (ECASS-1) pioneered the importance of assessing EIC to predict benefit from thrombolysis and introduced the “one-third” rule. A post hoc analysis suggested that the extent of EIC is an important predictor of the response to IV thrombolysis. In patients with a small (≤1/3 of the middle cerebral artery [MCA] territory) hypoattenuating area, thrombolysis increased the chance of good functional outcome (odds ratio [OR] 3.43; 95% confidence interval [CI] 1.61–7.33). The benefit was less clear for patients with absence of EIC (OR 1.27, 95% CI 0.82–1.95) or hypoattenuation involving greater than one-third of the MCA territory (OR 0.41, 95% CI 0.06–2.70). Increased risk for sICH was confirmed in secondary analysis of the ECASS-2 CT scans. However, despite evidence that the one-third MCA rule was a good prognostic marker, no statistical evidence of treatment effect modification was proven. In addition, volume estimation with the one-third rule was found in routine practice not to be reliable.
Given the difficulties with the reliability of the one-third MCA rule, the Calgary Stroke Program developed ASPECTS as a simple, reliable, and systematic approach to assessing EIC on noncontrast CT (NCCT). Early Ischemic Changes (EIC) on cerebral NCCT were initially defined as: (1) parenchymal hypoattenuation (gray-white indistinction or decreased density of brain tissue relative to attenuation of other parts of the same structure or of the contralateral hemisphere); and (2) focal swelling or mass effect (any focal narrowing of the cerebrospinal fluid spaces as a result of compression by adjacent structures).
Because isolated cortical swelling is associated with differential tissue outcomes and may represent actual penumbral tissue, EIC contributing to ASPECTS are defined as parenchymal hypoattenuation only. Parenchymal hypoattenuation can be focal hypodensity and/or loss of gray-white matter differentiation. Regions with isolated cortical swelling, that is, focal swelling without associated hypoattenuation on NCCT, do not contribute to ASPECTS.
Methodology
ASPECTS allots the MCA territory 10 regions of interest, which are weighted based on functional importance ( Fig. 1 ). Equal weighting is given to smaller structures (such as the internal capsule, basal ganglia, and caudate nucleus) as is given to larger cortical areas. ASPECTS methodology includes assessment of all axial cuts of the brain CT scan in two standardized levels of the MCA territory. The boundary of these two levels is the caudate head (see Fig. 1 ). Any ischemic lesion on axial CT cuts at the level of the caudate head or below is adjudicated to a ganglionic ASPECTS region (M1–M3, insula, caudate nucleus, lentiform nucleus, internal capsule); ischemic lesions above the level of the caudate head are adjudicated to a supraganglionic ASPECTS region (M4–M6). The caudate nucleus is assessed in both the ganglionic level (head of caudate) and supraganglionic level (body and tail of caudate) (see Fig. 1 ).
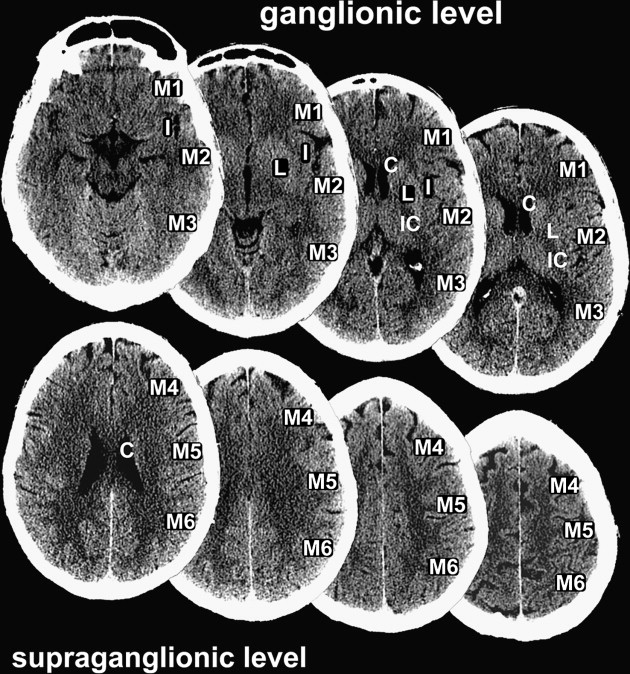
To compute the ASPECTS, a single point is subtracted from 10 for evidence of EIC in each of the 10 ASPECTS regions (see Fig. 1 ). A score of 10 reflects a normal CT scan, a score of 0 diffuse ischemic involvement throughout the complete MCA territory. To allow visualization of all ASPECTS regions in more than one slice, axial cuts with 4- to 5-mm slice thickness should be used. EIC should be visible on at least 2 adjacent cuts to ensure that a lesion is truly abnormal rather than a partial-volume effect. In addition, great care should be taken when comparing left and right hemispheres when patients are not adequately positioned in the CT scanner. Head tilt, motion artifact, and bone artifact are 3 of the commonest reasons for false-positive regional scoring with ASPECTS. The rule of thumb is that if there is doubt about a region, do not call it abnormal. Examples of typical ASPECTS patterns in patients with MCA M1- or M2-segment occlusions are presented in Figs. 2 and 3 .
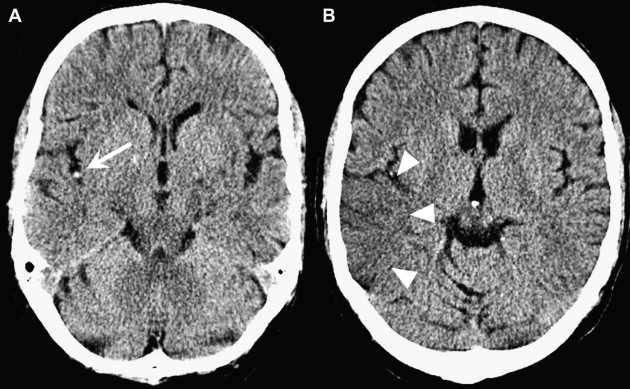
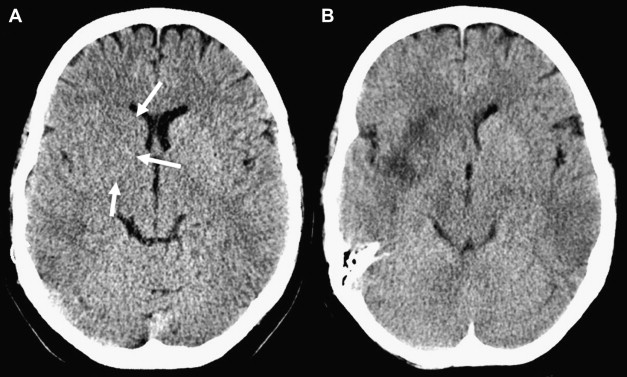
Does ASPECTS on NCCT at Baseline Predict Clinical Outcome in Patients with Acute Ischemic Strokes?
In the original ASPECTS study on patients thrombolyzed within 3 hours from symptom onset, a baseline ASPECTS value of 7 or below sharply discriminated patients who were highly unlikely to achieve an independent functional outcome. Since then, several studies have confirmed the prognostic value of ASPECTS in a 0- to 3-hour and 0- to 6-hour time window.
The prognostic information of ASPECTS was reproduced in the Canadian Alteplase for Stroke Effectiveness Study (CASES). Among 936 patients, the baseline NCCT ASPECTS score was a strong predictor of outcome, with lower scores implying a lower probability of an independent functional outcome (OR 0.81, 95% CI 0.75–0.87, per point ASPECTS decrease). However, with the larger number of patients studied in CASES, the prognostic information from ASPECTS was not dichotomous (as noticed in the smaller ASPECTS study), but near linear. In subsequent analysis, ASPECTS predicted functional outcome in a graded fashion, with a linear relationship for ASPECTS scores 6 through 10 and an ill-defined pattern for ASPECTS 0 through 5. With ASPECTS values 6 to 10, about 50% of patients experienced an independent functional outcome. By contrast, with an ASPECTS score of 0 to 3 the chance for an independent functional outcome was very low (about 15%). However, the ability of ASPECTS to discriminate individual outcomes was only modest (c-statistic = 0.63; 95% CI 0.59–0.67) and less than that of the baseline National Institutes of Health Stroke Scale (NIHSS) score (c-statistic = 0.71; 95% CI 0.67–0.74).
In an analysis of the Penumbra Pivotal Stroke Trial, good clinical outcome (modified Rankin Scale 0–2) was significantly greater in the ASPECTS >7 group when compared with the ASPECTS ≤7 group (risk ratio [RR] 3.3, 95% CI 1.6–6.8). No patient with an ASPECTS score of 4 or less had good clinical outcome.
Does ASPECTS on NCCT at Baseline Predict Response to Thrombolysis?
Although the extent of infarcted brain at baseline is a known prognostic factor in acute ischemic stroke, proof of treatment effect modification by imaging in acute ischemic stroke has been limited. This discrepancy between the proven role of baseline imaging as a prognostic factor and the inability to show effect modification with treatment is a central problem in our field. An oft-quoted explanation for this discrepancy is that the range of variation in patient outcome due to treatment effect is quite small when compared with the effect of imaging as a prognostic factor. Although this is true for large lesions where the prognosis is so poor that the effect of treatment may be negligible, it does not explain the lack of treatment effect modification in patients with small cores and large penumbra. Lack of uniformity in measurement is another possible explanation, and has plagued the technique of perfusion imaging and the mismatch hypothesis. There is, however, some data to suggest that such an interaction may exist with ASPECTS on NCCT ( Table 1 ).
| Study | Treatment Modality | Patient Selection Criteria | Main Findings |
|---|---|---|---|
| NINDS | IV, ≤3 h | No ICH on NCCT | Larger amount of benefit with higher ASPECTS values No thrombolysis effect modification |
| ECASS-2 | IV, ≤6 h | No ICH on NCCT EIC <1/3 MCA | Increased PH rate with ASPECTS ≤7 vs >7 No thrombolysis effect modification |
| PROACT-II | IA, ≤6 h | No ICH on NCCT M1 or M2 occlusion on DSA | Treatment effect modification by dichotomized ASPECTS (>7 vs ≤7) NNT = 3 with ASPECTS >7 |
| IMS-1 | IV-IA, ≤3 h | No ICH on NCCT EIC <1/3 MCA | Treatment effect modification by dichotomized ASPECTS (>7 vs ≤7) |
| Penumbra Pivotal Stroke Trial | IA alone or refractory to IV, <8 h | No ICH on CT, EIC <1/3 MCA, NIHSS ≥8 | Onset to recanalization time by dichotomized ASPECTS (>7 vs ≤7) effect modification |
Intravenous thrombolysis
In a post hoc analysis of the NINDS rtPA Stroke Study, ASPECTS on baseline NCCT dichotomized into greater than 7 versus 7 or less did not have a treatment-modifying effect on favorable functional outcome. However, higher ASPECTS values (ASPECTS 8–10) were associated with a greater extent of benefit from IV thrombolysis (absolute risk reduction 19.5% [10.2–28.8]; number needed to treat [NNT] = 5) and a trend toward reduced mortality. Mean final infarct volumes were also half as large in rtPA compared with placebo patients (7.8 mL vs 15.2 mL, respectively). Patients with a moderate extent of EIC (ASPECTS 3–7) still benefited from IV thrombolysis (absolute risk reduction 13.3% [0.3–26.3]; NNT = 8) but no mortality difference was seen. Final infarct volumes were 30% smaller in rtPA-treated patients (51.1 mL vs 66 mL, respectively). In the low ASPECTS group (ASPECTS 0–2) with extensive EIC no benefit of rtPA could be demonstrated. These patients had large mean final infarct volumes exceeding 200 mL. This extensive EIC group represented only 16 of 608 patients (2.6%) in the NINDS trial, thus limiting the group’s clinical relevance.
Effect modification of IV thrombolysis in a 0- to 6-hour time window has been addressed by applying ASPECTS to the ECASS-2 brain CT scans. The analysis also did not reveal a multiplicative thrombolysis-by-ASPECTS treatment interaction on functional outcome ( P = .29 for the interaction term). Moreover, this lack of a multiplicative thrombolysis-by-ASPECTS interaction was consistent for the 0- to 3-hour ( P = .89) and 3- to 6-hour ( P = .22) time window subgroups.
An inability to show treatment effect modification in these large IV thrombolytic trials could be because a large proportion of subjects had an ASPECTS of 10 and therefore possibly no occlusion or distal occlusions, EICs may not be sensitive enough within an early (3-hour) time window, and because the studies are underpowered to show such an effect.
Intra-arterial thrombolysis
In a post hoc analysis of the Prourokinase Acute Cerebral Infarct Trial II (PROACT-II) scans reevaluated for EIC using ASPECTS, a clear treatment interaction was identified. PROACT-II patients represented a fairly homogeneous cohort of patients with angiographically proven MCA M1- or M2-segment occlusions. Patients in the active treatment group received intra-arterial (IA) thrombolysis infusion with prourokinase proximal to the thrombus within 6 hours from symptom onset. Specifically, the reanalysis demonstrated that patients treated with IA prourokinase did better than controls only with a baseline ASPECTS greater than 7. The magnitude of benefit for these patients was large, with an NNT of 3 to yield functional independence at 90 days. Moreover, the benefit of IA thrombolysis progressively increased from ASPECTS 8 through 10 (RR 3.2 [95% CI 1.2–9.1] for ASPECTS >7; 7.5 [95% CI 1.1–51.4] for ASPECTS >8; and infinite for ASPECTS >9). In contrast, on average, patients with a baseline ASPECTS value ≤7 did not benefit from IA thrombolysis (RR 1.2, 95% CI 0.5–2.7).
CT scans of the Reevaluation of the Interventional Management of Stroke (IMS-1) study sought to determine whether ASPECTS could predict which patients would particularly benefit from combined IV-IA thrombolysis. IMS-1 used a safety and futility design, comparing historical control subjects from the NINDS rtPA Stroke Study. In IMS-1, a total of 80 patients were treated with IV rtPA (0.6 mg/kg) within 3 hours from symptom onset. At angiography, 62 received additional IA rtPA to a maximum dose of 22 mg. Patients with an ASPECTS score of greater than 7 were more likely to benefit from the combined IV-IA approach than from IV rtPA alone (based on matched subjects from the NINDS rtPA study ASPECTS analysis), with a NNT of 10. Patients with an ASPECTS score of less than 8 were less likely to benefit from combined IV-IA than from IV thrombolysis, and more likely to be harmed by interventional therapy. These data are evidence of both a qualitative and quantitative interaction effect.
Does ASPECTS Predict sICH Risk?
Numerous studies have demonstrated the association between the extension of EIC on NCCT and risk of hemorrhagic transformation after thrombolysis. However, a consistent EIC-by-thrombolysis interaction on sICH risk has not been established. In the original ASPECTS study, the authors found a 14-fold (95% CI 1.8–117) increased odds for sICH with ASPECTS scores of 7 or less versus greater than 7. In the secondary analysis of the NINDS-stroke trial, ASPECT scores of more than 7 and 3 to 7 carried a similar sICH rate of 5% and 4.5%, respectively. Only patients with very extensive EICs (ASPECTS 0–2) showed a trend toward increased sICH risk (20%; 95% CI 1.6–38.3). In secondary analysis of ECASS-2 with ASPECTS, an effect modification by ASPECTS greater than 7 versus 7 or less in predicting radiologically defined parenchymal hematoma but not sICH was demonstrated. When dichotomized ASPECTS was applied to the PROACT-II CT scans, ASPECTS 7 or less was associated with doubled risk for treatment-related sICH compared with scores of more than 7 (15.4% vs 7.7%, respectively). However, no IA-thrombolysis-by-ASPECTS interaction on sICH risk was demonstrated.
The extent of EIC using ASPECTS is associated with the risk for thrombolysis-related hemorrhagic transformation. From current data, dichotomized ASPECTS modifies the risk for thrombolysis-related radiologically defined parenchymal hematoma but not clinically defined sICH.
Does ASPECTS on NCCT at Baseline Help Predict the Time Window Available for Recanalization?
Time has been shown to be an important modifier of intravenous thrombolytic treatment effect in randomized trials. In an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials, Lees and colleagues show benefit with IV tPA in patients treated within 4.5 hours of symptom onset. By demonstrating an effect of “onset to treatment time” on clinical outcomes in the treatment group vis-a-vis placebo, they show the time-dependent nature of treatment benefit and risk. This makes biologic sense, as progression to irreversible infarction in the penumbra is a time-dependent phenomenon and early treatment aimed at recanalization should modify this process. This penumbral hypothesis raises the possibility that patients with smaller areas of infarction at baseline imaging would benefit most, and as infarct core increases in size the time window available shrinks rapidly. This interaction between baseline CT scan and onset to recanalization time is particularly appealing given our understanding of modern imaging.
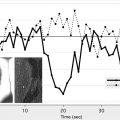
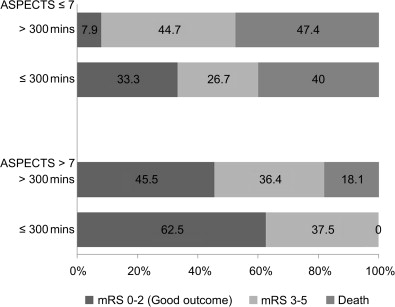
A post hoc analysis of the IMS-1 study failed to show an interaction between ASPECTS on NCCT at baseline and time to treatment. However, an analysis of data from the Penumbra Pivotal Stroke Trial (a prospective, single-armed 125-patient study designed to assess the safety and efficacy of the “Penumbra” system for removing thrombi in acute ischemic strokes) showed that in the ASPECTS >7 group, 62.5% of patients with onset to recanalization time of 300 minutes or less had good clinical outcome when compared with 45.5% patients with either onset to recanalization time of longer than 300 minutes or nonrecanalizers. In the ASPECTS ≤7 group, the corresponding figures were 33.3% with onset to recanalization time of 300 minutes or less versus 7.9% in the >300 minutes or nonrecanalizer group ( Fig. 4 ). After adjusting for baseline stroke severity, there was evidence of an ASPECTS-by-onset to recanalization time interaction ( P = .066) in the multivariable model. The direction of interaction was such that among patients with ASPECTS greater than 7, the relative effect of onset to recanalization time (≤300 minutes or >300 minutes) in predicting outcome was small. Among patients with ASPECTS of 7 or less, only those with an onset to recanalization time of 300 minutes or less had some chance of achieving a good functional outcome. Patients with an unfavorable scan (ASPECTS ≤7), with late recanalization, did poorly. At any treatment time point, if there are extensive changes on CT scan (ASPECTS ≤4), endovascular therapy simply does not help.
Reliability of ASPECTS Compared with the One-Third Rule
The original ASPECTS study compared reliabilities of the one-third rule and ASPECTS. ASPECTS dichotomized at >7 versus ≤7 consistently demonstrated higher agreement (κ = 0.71–0.89) as compared with the one-third rule (κ = 0.52–0.64) among stroke neurologists, radiology trainees, and experienced neuroradiologists. ASPECTS was also reliable in real time when stroke neurologists and stroke fellows prospectively recorded NCCT ASPECT scores in the emergency department.
However, Mak and colleagues found a higher reliability with the one-third rule (κ = 0.49) compared with dichotomized ASPECTS (κ = 0.34) among reviewers from different specialties. The investigators attributed this result, in part, to a more systematic approach to the one-third rule by applying the worksheet from the ATLANTIS/CT summit criteria. When experienced stroke neurologists used a more systematic approach to the one-third rule with the ICE method (Idealize the MCA territory; Close a geometric figure around the areas of hypodensities; Estimate the ratio of the 2 geometric figures), kappa statistics were similar for the one-third rule and dichotomized ASPECTS (κ = 0.7 for both).
A more systematic approach may improve reliability of the one-third rule. Such systematic approaches are essentially the same as ASPECTS. In fact, dichotomized ASPECTS and the one-third rule may provide similar information. An ASPECTS cutoff of 6 or less had 94% sensitivity and 98% specificity, respectively, to estimate MCA infarction greater than one-third. In the ECASS-2 study baseline CTs independently scored to have EIC in more than one-third of the MCA territory, the median ASPECTS score was 4. Thus, the authors have recommended that an ASPECTS of less than 5 is equivalent to more than one-third MCA infarction. A major advantage of ASPECTS is its increased sensitivity for EIC in the MCA territory.
ASPECTS on CT Angiography Source Image
Hypoattenuation on NCCT brain is caused by a shift in brain tissue water content secondary to significant ischemia (brain parenchyma with blood flow <12 ml/100 g/min). Animal experiments suggest that a 1% increase in brain tissue water content results in a drop of 1.8 Hounsfield Unit in attenuation. For hypoattenuation to be visible to the human eye, there has to be a large shift in water uptake in ischemic tissue. This process is dependent on time and degree of ischemia and therefore is better appreciated at later time intervals from stroke onset. On the other hand, hypoattenuation on CT angiography source image (CTA-SI) (if image is in the venous phase) is an indicator of reduced cerebral blood volume in the area of ischemia and is not as time dependent as ischemia on NCCT. Areas of hypoattenuation on CTA-SI correlate well with lesions on diffusion-weighted imaging (DWI) and final infarcts. Applied on ASPECTS, CTA-SI has improved the prediction of final infarct size and clinical outcome compared with NCCT ( Fig. 5 ).
ASPECTS on Multimodal CT and Magnetic Resonance Imaging
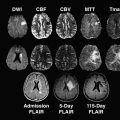
Although ASPECTS was originally designed for use with NCCT, recent studies indicate its practicability with perfusion CT (CTP) and multimodal magnetic resonance (MR) imaging with DWI and perfusion-weighted imaging (PWI). MR) with diffusion-weighted sequences is more sensitive for EIC than NCCT, particularly in patients presenting with minor symptoms. ASPECTS on DWI may therefore overestimate EIC with prognostic relevance. As a consequence, the authors do not advocate use of ASPECTS on DWI in patients with transient ischemic attack or minor stroke. However, in patients presenting with disabling symptoms within 6 hours from symptom onset, the ASPECTS differences between NCCT and DWI in visualizing early infarction were small. Consequently, ASPECTS applied to DWI was a reliable tool for predicting poor outcome in patients thrombolyzed within 3 hours from symptom onset in a recent study, with a DWI ASPECTS of 5 or less being an independent predictor of poor functional outcome (OR 33.4; 95% CI 2.7–410.8; P = .0062). In a large IV tPA registry, a pretreatment ASPECTS of 7 or more on DWI was related to good clinical outcome (OR 1.85; 95% CI 1.07–3.24) when compared with an ASPECTS of 4 or less, which was related to death (OR 3.61; 95% CI 1.23–9.91), and ASPECTS 5 or less, related to symptomatic ICH (OR 4.74; 95% CI 1.54–13.64).
Efforts to use the penumbral hypothesis to identify a subgroup of patients who would benefit with thrombolysis has not yielded expected results. Lack of uniformity in measurement of perfusion parameters is an explanation. In clinical practice, rapid and reliable assessment tools to define a CTP- or MR imaging-based mismatch are required. However, purely subjective assessment of a DWI/PWI mismatch has poor interrater reliability and volumetric assessment is not clinically practical. ASPECTS mismatch on DWI and PWI is a rapid and reliable alternative. A linear correlation between DWI ASPECTS and DWI lesion volume has previously been shown. In a post hoc analysis of the EPITHET data, Butcher and colleagues found 78% (interrater range 72%–84%) sensitivity and 88% (interrater range 83%–90%) specificity for a “DWI-ASPECTS minus PWI-ASPECTS” difference of 2 or more to predict a 20% DWI/PWI mismatch. Applied to CTP, Lin and colleagues have reported 84% (95% CI 65.5%–93.5%) sensitivity and 100% (95% CI 65.9%–100%) specificity of a cerebral blood volume (CBV) ASPECTS minus mean transit time (MTT) ASPECTS difference of 1 or more to identify a volumetric CBV/MTT mismatch of 20% or more. In a study by Parsons and colleagues, ASPECTS applied on CBV parameter maps was more accurate in predicting irreversibly injured tissue and clinical outcome than NCCT or CTA-SI. Moreover, ASPECTS on cerebral blood flow (CBF) parameter maps predicted tissue at risk for infarction without reperfusion (ie, the ischemic penumbra) in this study.
Clinical diffusion mismatch (CDM) has often been used as an alternative to DWI/PWI mismatch in identifying patients with brain tissue at risk of infarction. Volumetric assessment of DWI limits clinical applicability of this paradigm. In a consecutive series of 71 patients with anterior circulation strokes treated with IV tPA, Terasawa and colleagues showed that CDM determined using DWI ASPECTS (NIHSS ≥8 and DWI ASPECTS ≥7) is associated with neurologic improvement when compared with a group without.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree