CHAPTER 20 Atherosclerosis and the Chronology of Infarction
DOLICHOECTASIA
Epidemiology
Dolichoectasia is found in fewer than 10% of patients. Its etiology is unknown but appears to be related to aberrant vascular remodeling. It is associated with traditional cardiovascular risk factors including hypertension, old age, and male sex, but also nonatherosclerotic diseases.1 Genetic associations are common in younger patients and include Marfan syndrome, Ehlers-Danlos syndrome, human immunodeficiency virus, Fabry’s disease, sickle cell disease, and α-glucosidase deficiency.
Clinical Presentation
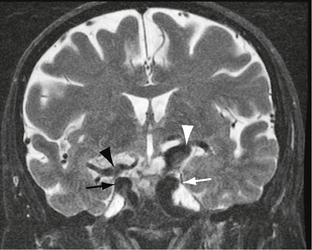
The vertebrobasilar arteries are affected most often, with less frequent involvement of the terminal internal carotid artery (ICA) and proximal middle cerebral artery (MCA). Dolichoectasia may also be associated with parenchymal mass effect, neurovascular impingement syndromes including trigeminal neuralgia and tinnitus, aneurysm formation, subarachnoid hemorrhage, and hydrocephalus. Supraclinoid lesions may impinge locally on the optic nerves and chiasm (Fig. 20-1). Focal fusiform aneurysmal dilatations are not uncommon. In older patients, dolichoectasia has been associated with severe small vessel ischemic change, lacunar infarcts, and état criblé but not carotid atherosclerosis.1a It is unclear as to what extent dolichoectasia contributes to white matter change or whether it is part of a more widespread vasculopathy affecting small and large intracranial vessels.
CT and MRI
Both CT and MRI reveal tortuous dilated vessels, which often impinge on and mold the medulla and pons (Fig. 20-2). Loss of signal void may be apparent on standard MRI. Intravascular contrast enhancement may be seen after contrast agent administration for the same reason (Fig. 20-3). Smoker’s criteria for basilar dolichoectasia includes artery diameter, laterality of basilar trunk, and height of basilar bifurcation.1
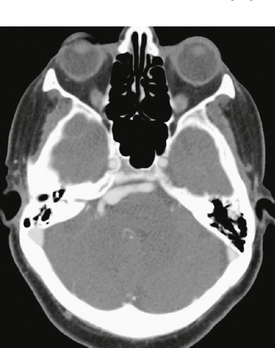
FIGURE 20-2 Enhanced axial CT at the level of the pons. There is a dolichoectatic right vertebral artery demonstrating variable dilatation that is distorting the pons.
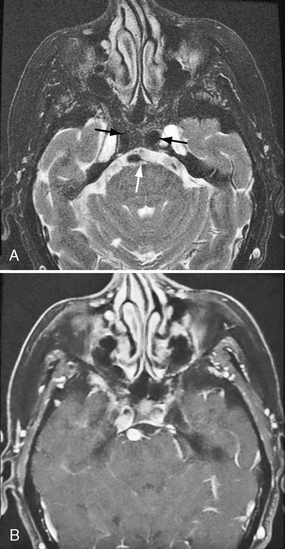
FIGURE 20-3 Fat-saturated axial T2W (A) and postcontrast T1W (B) MR images (same case as Figs. 20-1 and 20-2). Note the dilated bilateral cavernous carotid segments and vertebral artery (arrows). There is a conspicuous absence of flow voids after contrast agent administration owing to hemodynamic disturbances.
NEUROVASCULAR COMPRESSION SYNDROMES
The prototypes of neurovascular compression syndromes actually are aneurysms and pulsating masses that can directly affect cranial nerves. For example, optic nerve dysfunction can be caused by aneurysms of the cavernous and ophthalmic regions. Aneurysms, arteriovenous malformations, and tumors are, however, found as the cause for neurovascular syndromes in only 2% of cases.
The success reported in several case series provides compelling evidence for the existence of neurovascular compression and for patient improvement after treatment.2,3 However, there is extreme variation of both the arterial and venous structures within the posterior fossa. Up to one third of asymptomatic patients may have imaging findings suggestive of cranial nerve/vascular contact.4 Therefore, imaging criteria have evolved to permit more specific diagnosis of symptomatic neurovascular compression. These include the distortion of the appropriate cranial nerve by a vessel passing perpendicular to it at the nerve root entry/exit zone.5 No prospective studies exist to assess the reliability of MRI for the diagnosis of neurovascular compression syndromes. Sensitivities and specificities for case series range from 44% to 100% and 50% to 100%, respectively. The lack of randomized trials with comparative outcomes (and for the patterns of treated normal anatomic variations) adds further uncertainty as to which patients should be submitted to surgery based on imaging.
Trigeminal Neuralgia
Pathophysiology
The pathogenesis is thought to be secondary to aberrant impulses, ephaptic cross talk, and conduction block secondary to neural injury. Neurovascular compression is implicated in the majority of cases (Fig. 20-4), but primary demyelination, tumor infiltration, amyloid, infarction, cavernous malformations, and idiopathic causes are also implicated. Familial cases have been described.
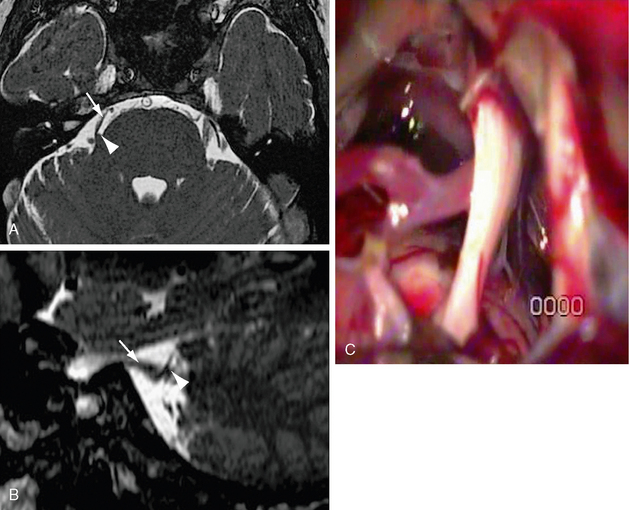
FIGURE 20-4 A, Axial FIESTA with (B) oblique sagittal reformat centered on the trigeminal level. There is attenuation and distortion of the right trigeminal nerve (white arrow) by the right superior cerebellar artery (white arrowhead), which passes perpendicularly across the nerve entry zone. C, Operative microscopic image demonstrating attenuation and pallor of the cisternal portion of the trigeminal nerve. A vascular structure crosses the nerve inferiorly.
(C, Courtesy of Milan Spaic, MD, Military Medical Academy, Serbia.)
Pathology
Histopathologically, demyelination extending less than 2 mm from the zone of vascular contact is reported.6 Demyelinated axons are in close apposition with no intervening glial processes. Electron microscopy demonstrates a combination of demyelination and remyelination. The trigeminal ganglion may show degenerative hypermyelination and microneuromas.
Imaging
Trigeminal neuralgia remains a clinical diagnosis. Microvascular compression is considered to be the major cause, so decompressive surgery is the mainstay of therapy. Other procedures to ablate the affected root of the fifth nerve, such as partial rhizotomy, radiofrequency thermocoagulation, stereotactic radiosurgery, and glycerol gangliolysis, are also performed.7 Pharmacotherapy is a further option. Imaging studies are indicated to exclude other lesions, such as aneurysm, arteriovenous lesion, schwannoma, meningioma, or other structural lesions in the cerebellopontine angle or Meckel’s cave. A vascular study may have little influence on management decisions,4 but MRI with a volumetric study and MRA with or without gadolinium are used. Diffusion tensor imaging has also emerged as a promising tool in assessing nerve integrity, showing decreased fractional anisotropy on the affected side.7a
Other Neurovascular Syndromes
A number of other syndromes have been attributed to neurovascular compression, including superior oblique myokymia (trochlear nerve), geniculate neuralgia (nervus intermedius), vestibular paroxysmia (vestibulocochlear nerve), spontaneous gagging (vagus nerve), and spasmodic torticollis (accessory nerve).
FIBROMUSCULAR DYSPLASIA
Clinical Presentation
Fibromuscular dysplasia is often asymptomatic. However, patients are predisposed to dissection and aneurysm formation with rupture (Fig. 20-5). Up to 15% of cervical artery dissections are secondary to fibromuscular dysplasia, but the risk of dissection in FMD patients is unknown.7b Vessel tortuosity and wall thickening may impair flow, resulting in transient ischemia attack (TIA) or ischemic stroke. Although the natural history is unknown, progression is reported in up to 25% of patients.
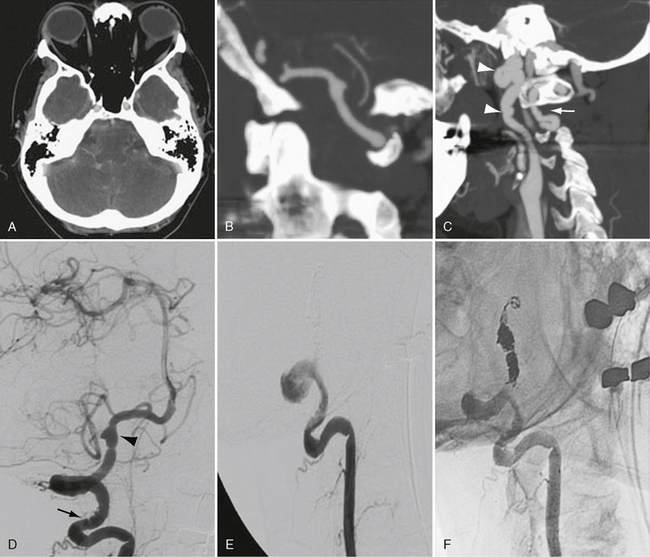
FIGURE 20-5 Rupture of a dissecting aneurysm secondary to fibromuscular dysplasia. A to C, Postcontrast CT and CTA rotational and sagittal maximal intensity projection images demonstrate subarachnoid hemorrhage from a dissecting aneurysm of the vertebral artery. Vertebral artery irregularity is present between C1 and C2 (arrow). The distal internal carotid artery demonstrates marked redundancy and beaded irregularity secondary to fibromuscular dysplasia (arrowheads). D to F, Cerebral angiography confirms cervical vertebral artery beading (arrow), a concentric band of narrowing within the cisternal vertebral segment, and a dissecting aneurysm inferior to the posterior inferior cerebellar artery origin (arrowhead). The patient was treated by ipsilateral vertebral artery coil occlusion across the level of the dissection. She had full recovery with no neurologic deficit on 1-year follow-up.
The extracranial ICAs are involved in 95% of cases. Bilateral disease occurs in 60% to 80%. The carotid bifurcation and proximal ICA are frequently spared, distinguishing fibromuscular dysplasia from atherosclerotic disease (Fig. 20-6). The vertebral arteries are affected at the level of C2 in 12% to 43% of cases with sparing of the origins and proximal portions of the vessels. Intracranial involvement is rare and most often from extracranial disease extension, although isolated intracranial FMD has been reported. Systemic vessels affected include renal, subclavian, iliac, and mesenteric arteries.
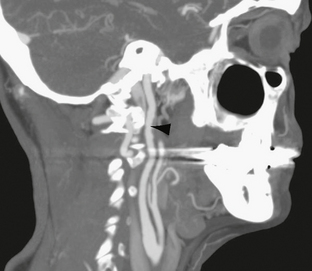
FIGURE 20-6 Rotational sagittal maximal intensity projection. The upper carotid artery shows irregularity of the wall with alternating dilatation and narrowing consistent with beading. There is greater involvement of the anterior wall, where diverticulation can be appreciated (arrowhead). The carotid bifurcation is normal.
Pathology
Fibromuscular dysplasia is subdivided according to medial, intimal, or adventitial involvement. Three distinct histologic types of medial dysplasia are described. Medial fibroplasia is most common (80%). Perimedial fibrosis occurs in 10% of patients, usually in younger women. The characteristic gross pathologic appearance is that of beading, although the beads are fewer in perimedial fibrosis. Regions of focal dilatation may be larger than the original luminal diameter. Medial hyperplasia is least common, resembling intimal fibroplasia. Intimal fibroplasia occurs in 10% of cases. Discrete bands of narrowing or tubular stenosis may occur (Fig. 20-7). Adventitial fibroplasia occurs in less than 1% of cases.
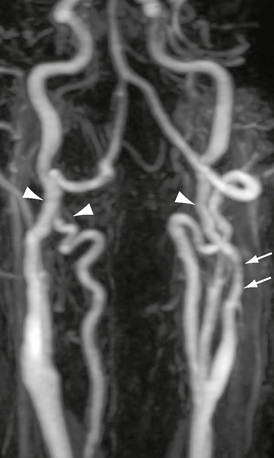
FIGURE 20-7 Rotational maximum intensity projection contrast-enhanced MRA. There is marked bilateral carotid and vertebral artery irregularity with subtle beaded appearance and modest redundancy (arrowheads). The left carotid artery demonstrates tubular narrowing above the carotid bifurcation (arrows).
Imaging
CTA and MRA
CTA and MRA have evolved as cross-sectional techniques and routinely detect arterial changes consistent with fibromuscular dysplasia (see Figs. 20-6 and 20-7). No published comparisons of noninvasive techniques for the diagnosis of fibromuscular dysplasia are found. Comparisons from the renal literature demonstrate higher accuracy for CTA and MRA than for transcranial Doppler ultrasonography. It can be inferred from experience with carotid atherosclerotic disease that conventional angiography may be reserved for cases in which CTA and MRA are equivocal or prior to transluminal intervention.
Special Procedures
Conventional angiography remains the gold standard. Angiographic appearances are typical, with 80% to 90% demonstrating alternating dilatation and narrowing producing a “string of beads” or “concertina” appearance (type 1 lesion) (see Figs. 20-5 and 20-6). Diffuse tubular narrowing is seen in 5% to 10% of cases (type 2 lesion) (see Fig, 20-7). The appearances may be difficult to distinguish from dissection, especially because dissection frequently coexists in fibromuscular dysplasia. Rarely, unilateral vessel wall involvement is seen. These lesions resemble diverticula (see Fig. 20-6), may be difficult to distinguish from atherosclerotic disease, and may be a source of thomboembolism.8 A web-like defect at the origin of the internal carotid artery is also rarely described (more commonly in black patients) and thought to be related to intimal FMD.7b
ATHEROSCLEROTIC DISEASE
Epidemiology
Atherosclerotic disease accounts for approximately one third of all cases of cerebral infarcts. Ten percent of such cases are caused by carotid stenosis that is amenable to revascularization. Carotid stenosis of more than 50% is found in 10% to 15% of patients with ipsilateral ischemic stroke and TIA, respectively.9,10 Imaging is, therefore, central to the prevention and treatment of stroke. Symptomatic disease is commonly secondary to atheroembolus and less commonly secondary to distal hypoperfusion caused by stenosis. The risk of atherosclerotic lesion progression is 9.3%. Numerous imaging-detectable risk factors impact on the annual stroke risk and should be actively sought. The two most important factors are degree of stenosis and symptom status. North American Symptomatic Carotid Endarterectomy Trial (NASCET) reported a threefold annual risk of stroke for symptomatic stenosis greater than or equal to 90% compared with 70% to 79% (11% and 35%, respectively). The stroke risk for near occlusion, however, is low (11%). Absence of symptoms decreases the risk significantly. Asymptomatic annualized stroke risk is 1.0% to 2.4% for 60% to 99% stenosis. The Asymptomatic Carotid Surgery Trial also found no increased stroke risk with increasing severity of stenosis. Imaging-detectable risk factors include plaque morphology, prior silent stroke, the presence of contralateral or intracranial disease, and intracranial collateral status.
Pathophysiology
Atherosclerosis is a complex disease with a multifactorial pathogenesis. It is believed to result from the interaction among endothelial cell injury, lipid accumulation, smooth muscle proliferation, plaque formation, inflammatory cell recruitment, matrix synthesis, and intimal neovascularization.11–14 Savory15 first noted an association between carotid artery disease and strokes in 1856. Subsequent pathologic series confirmed the presence of intracranial carotid atheroembolic material in stroke patients.16 Fisher,17 evaluating carotid arteries pathologically, concluded that intraplaque hemorrhage, plaque ulceration, and luminal thrombosis were the main substrates for cerebral embolism.
Pathology
Atherosclerosis is the most common pattern of arteriosclerosis. It affects arteries of medium-to-large caliber.18 Other patterns are Mönckeberg medial calcific sclerosis and arteriolosclerosis.18 Mönckeberg medial calcific sclerosis refers to calcific arterial deposits usually in people aged 50 years or older. Arteriolosclerosis affects small arteries and arterioles with arteriolar wall thickening usually associated with diabetic and hypertensive patients.
The components of atherosclerotic plaques can be divided into four categories19: (1) lipids (including crystalline cholesterol, phospholipids, and cholesteryl esters), (2) connective tissue matrix components (including collagen, proteoglycans, and fibronectin), (3) cellular components (e.g., monocytes, macrophages, T lymphocytes, and smooth muscle cells), and (4) thrombotic materials (platelets and fibrin). The components are present in varying proportions throughout the life of the plaque. The specific composition of atherosclerotic plaques forms the basis for a variety of classification schemes for plaques.
The American Heart Association (AHA) Committee on Vascular Lesions classification, or Stary classification, is the most widely used scheme. It attempts to correlate histologic lesion appearance with imaging appearance.20–23 Plaque histologic/histochemical composition and cellular/matrix components form the basis for classification. Eight plaque categories (types I-VIII) make up this classification.20,22 Type I to III lesions may regress to a normal phenotype. Type IV to VIII plaques that progress to lesions are termed atheromas or advanced atherosclerotic lesions. Up to type V, remodeling of the arterial wall24 occurs with outward displacement of the wall in response to increased atheroma. Initially, an eccentric pattern of remodeling occurs.24 Subsequently, collagen layer formation limits outward displacement, resulting in luminal narrowing, hemorrhage and ulceration (type VI), calcification (type VII), and fibrosis (type VIII). The scheme has been criticized for not including plaque features such as plaque erosion and thin-cap fibroatheroma, features that are addressed by other schemes beyond the scope of this text.19,25
Imaging
Ultrasonography
B mode imaging may detect plaque irregularity, ulceration, and echogenicity, which are markers of stroke risk. Hyperechoic areas are shown to represent plaque fibrosis/microcalcifications. Hypoechoic areas are associated with hemorrhage, thrombosis, and/or lipids in the vessel wall.26,27 Intimal thickness has been used as a marker of atherosclerosis. However, an increased intimal thickness does not differentiate between atherosclerotic disease and other processes such as intimal or medial infiltration/hypertrophy, which might increase this measurement.28,29 Intravascular ultrasound enables high spatial resolution imaging of the arterial wall (100-250 μm); however, low sensitivities for thrombus and lipid-rich lesion detection have been reported.30
Numerous diagnostic criteria exist for the diagnosis of carotid stenosis. A consensus group recommended that the peak systolic velocity (PSV) should be the main determining parameter. In the presence of tandem lesions, contralateral high-grade stenosis, and hyperdynamic or hypodynamic cardiac pulsation, the PSV may be inaccurate. Under such conditions ICA/common carotid artery (CCA) PSV and ICA end-diastolic volume (EDV) may be helpful.31 A PSV, ICA/CCA PSV ratio, and EDV less than 125 cm/s, 2 cm/s, and 40 cm/s, respectively, is considered normal. PSV, ICA/CCA PSV ratio, and EDV of 125 to 230 cm/s, 2 to 4 cm/s, and 40 to 100 cm/s, respectively, is consistent with 50% to 69% stenosis. A 70% and greater stenosis is diagnosed when PSV, ICA/CCA PSV ratio, and EDV are greater than 230 cm/s, 4 cm/s, and 100 cm/s, respectively. In all cases the PSV is the primary parameter and the ICA/CCA PSV ratio and EDV are additional parameters.31 Applying different thresholds, the sensitivity of Doppler ultrasonography in diagnosing a stenosis greater than or equal to 70% relative to conventional angiography is reported as 77% to 98% in a study of 1006 carotid arteries. Specificity is 53% to 82%. Agreement with conventional angiography for stenosis of greater than or equal to 70% is 96%. However, agreement with conventional angiography for less severe stenosis is poor (45%).32 Cervical artery examination may be supplemented by transcranial ultrasound to screen for intracranial atherosclerosis and to assess for hemodynamic changes resulting from hemodynamically significant extracranial stenosis. Embolic signal detection by transcranial examination appears to predict stroke risk in acute stroke, in symptomatic carotid stenosis, and post-operatively following carotid endarterectomy.32a
The main limitation of ultrasonography is wide variation in agreement of parameters that are indicative of carotid stenosis. The well-known high interoperator dependence and variation between machines and manufacturers result in a lower reproducibility.28 Heavily calcified plaques, tortuous vessels, hairline residual lumen, and high carotid tandem lesions may not be assessable. Only the cervical portion of the ICA is evaluated if the study is not supplemented by transcranial ultrasound. Contralateral carotid occlusion may increase peak systolic velocity and may lead to stenosis overestimation. Despite these limitations, ultrasonography remains the most widely used modality for screening of carotid disease. Although it is often the only examination performed, several studies confirm the benefit of a combination of noninvasive tests.33,34 One study compared ultrasonography and MRA to conventional angiography, reporting a misclassification rate of 28% and 18% for ultrasonography and MRA, respectively. Misclassification is reduced to 6% to 11% when two noninvasive modalities are combined.33,35 We recommend the use of ultrasonography for screening with a second noninvasive confirmatory test. Our preference is CTA. In the case of agreement, no further investigation is required. Disagreement necessitates conventional angiography rather than a third noninvasive test.
CTA
The performance of CTA compared with digital subtraction angiography (DSA), MRA, and ultrasonography has been studied in multiple small series. A meta-analysis36 of studies prior to 2003, including only single-slice scanners, found a sensitivity and specificity for detection of 70% to 99% stenosis of 85% and 93%, respectively. For occlusion, values of 97% and 99% are reported. A recent small study utilizing a four-slice scanner showed sensitivity, specificity, and accuracy of 95%, 93%, and 94%, respectively, for stenosis of 50% or more. There have been rapid technologic software and hardware advances since these studies, and now 64- and 320-slice multidetector scanners are in routine clinical use. These scanners have higher through-plane resolution because of smaller detector size. Together with reduced scan times, the extent of coverage is improved and there is less venous contamination. Reversal of scan direction may further limit venous contamination, but in our experience this is seldom a problem.37 A recent study utilizing an 8-slice scanner found that CTA underestimated the degree of stenosis relative to DSA but achieved a high correlation of 0.95. Interobserver agreement was 3.9% for DSA and 5.8% to 5.9% for CTA using manual or automated measures, respectively.38 In practice, non-invasive cross-sectional imaging has replaced conventional angiography for this purpose. It is unlikely that a large study comparing newer generations of multidetector CTs to DSA will ever be published.
In the acute setting, the differentiation of near occlusion (string sign) from a total occlusion is of importance because a very high-grade stenosis can be addressed with either surgical or endovascular procedures, whereas a total occlusion cannot unless occluded with fresh thrombus. CTA appears almost as accurate as DSA for differentiating carotid occlusion from near occlusion, and significantly superior to carotid Doppler ultrasound.38a In this setting, a major caveat of CTA is the possibility of nonopacification of the distal ICA secondary to very slow flow, therefore mimicking a carotid occlusion. This problem can be overcome by delayed CTA acquisition.38b Furthermore, in near occlusion, the distal carotid artery will collapse secondary to severely reduced poststenotic arterial pressure. This may lead to underestimation of the stenosis when using NASCET-style measurements unless this pitfall is recognized.38c
The method of data reconstruction significantly alters the performance of both CTA and MRA.39 In our experience, axial source images are the most accurate and reproducible method for stenosis measurement. Although calcium complicates measurement on axial imaging, a wider window (WW 900, WL 275) is usually sufficient to overcome this. Multiplanar reconstructions (MPR) and especially maximum intensity projection (MIP) techniques are less reliable in the presence of calcification. Dual energy CT is a novel approach to remove calcified plaques from the CTA images.39a,39b A thorough review of different processing techniques is described elsewhere.39 With proper attention to detail we have found that axial source images are equivalent to axial oblique images (performed tangential to the vessel lumen). We use a combination of MPR and axial source images to understand the position and course of the lumen through the axial plane before stenosis measurement are made on axial images (Fig. 20-8). An advantage of cross-sectional imaging is that NASCET style measurements are no longer needed. Stenosis can be described as a cross-sectional area or single proximal measurement. There is an excellent agreement for a single proximal measurement with a NASCET style measure40 and cross-sectional area.41
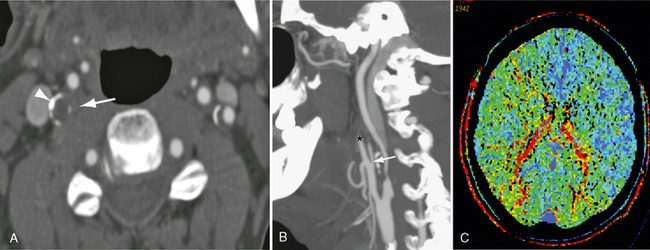
FIGURE 20-8 A patient presented with transient left-sided weakness consistent with a transient ischemic attack. Reconstructed 0.63-mm axial CTA (A) and sagittal MIP (B) images demonstrate bilateral carotid bifurcation atherosclerotic changes. Low-density atheroma and peripheral calcification (arrowhead) surround both vessels. The residual right lumen measures 0.1 mm (white arrow). The distal carotid is reduced relative to the contralateral carotid, although it is larger than the ipsilateral external carotid artery (asterisk). The appearances are consistent with a high-grade stenosis. C, Mean transit time map demonstrates prolongation of transit time within the distribution of the right carotid artery.
Multidetector CT has been used to image atherosclerotic plaques in the coronary and carotid arterial circulations.42,43 Plaques may be classified as fatty (Fig. 20-9), mixed or calcified, and stratified by Hounsfield unit (HU) attenuation.42,44 In vitro and in vivo studies have produced mixed results for CTA plaque characterization,45–47 favoring MRI for this application.
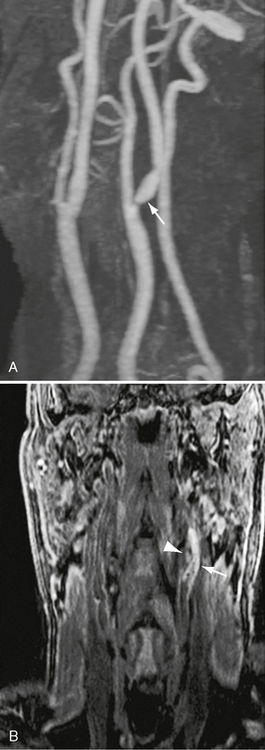
FIGURE 20-9 A and B, Magnified rotational maximum intensity projection contrast-enhanced MRA to illustrate cervical findings. There is a high-grade left ICA stenosis. A flow void at the stenosis site makes a direct measurement impossible (arrow). A gradient coronal 3D T1W spoiled gradient with fat saturation confirms the presence of a complicated or hemorrhagic plaque (arrowhead).
MRI
Advances in MRI sequences, coils, and higher gradient scanners have resulted in improved contrast and spatial and temporal resolution. Whereas TOF studies dominated the earlier literature, increasingly contrast-enhanced MRA is performed. Reflected in these changes is the improved performance of MRA when compared with DSA. A meta-analysis of MRA studies between 1990 and 1999 reported that MRA appeared accurate but that interpretation was hampered by study heterogeneity. More recent studies report high sensitivity, specificity, and accuracy for both 3D TOF MRA and contrast-enhanced MRA.48
A recent study comparing TOF MRA and contrast-enhanced MRA with conventional angiography and 3D DSA in 98 carotid arteries found high agreement for all techniques.49 The sensitivity/specificity for contrast-enhanced MRA and TOF MRA using rotational angiography as a reference standard was 100%/90% and 95.5%/87%, respectively. The specificity was lower when compared with conventional angiography because of the inherent underestimation of this technique relative to DSA. TOF sequences are longer than contrast-enhanced MRA and suffer from dephasing secondary to in-plane flow and flow-related artifacts such as turbulence and patient movement. Contrast-enhanced MRA overcomes many of these limitations with rapid scan times, greater coverage, and improved contrast to noise. Administration of contrast agents such as gadobenate dimeglumine with higher T1 relaxivity and signal intensity enhancement further improves contrast resolution.
Similar to CT, MRA data may be displayed in a variety of ways, influencing the test performance. In general, review of source data together with other reformatted images is the best approach, with MPR demonstrating higher concordance than MIP images.39 We routinely acquire a venous-phase CT after CTA, which allows distinction of near occlusion from total occlusion. In our experience, acute carotid occlusion will often show peripheral enhancement of the carotid, the lumen being filled with nonenhancing thrombus. Advantages of MRI are noninvasiveness, high contrast resolution, and lack of ionizing radiation. However, MRI is still not widely available, is expensive, and requires a longer study time than CT. The administration of gadolinium compounds should be avoided in patients with acute renal failure or in renal dialysis patients because of the association with nephrogenic systemic fibrosis.50 Six to 10 percent of patients are unable to undergo MRI studies.51 MRI contraindications including metallic implants and clips and patient claustrophobia are the most common reasons.
Recent advances in MRI have made it an emerging modality for assessment of atherosclerotic carotid plaques (Fig. 20-10).52–55 The development of carotid phase-array coils has allowed increasing signal-to-noise and contrast-to-noise ratios and submillimeter voxel-size imaging of carotid atherosclerotic plaques.54,56–60 Plaque composition is an important factor influencing plaque rupture.61,62 Good correlation between MRI and ex vivo histopathology specimens is reported.53,54,63–66 Imaging of the vessel wall area has also been shown to be feasible with high-resolution carotid plaque imaging. Both 2D67 and 3D68–70 sequences have been described. A recent study by Mani and coworkers71 demonstrated good correlation between black-blood MRI carotid imaging and intimal thickness for the detection of carotid wall area, wall thickness, and plaque index. Contrast-enhanced studies have also been very useful in assessing plaque inflammation, with the degree of enhancement seen in the fibrous regions of the plaque correlating with the amount of neovasculature present in the plaque.72 The two most important features of a vulnerable plaque that can be identified by MR are adventitial enhancement and intraplaque hemorrhage.72a
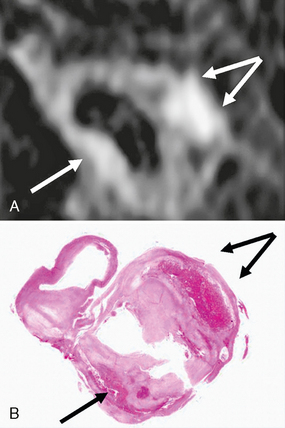
FIGURE 20-10 MRI (3D T1W fat suppressed spoiled gradient echo sequence) of intraplaque hemorrhage in a 74-year-old man with symptomatic high-grade carotid stenosis. A, MR image at the level of the carotid bifurcation. Intraplaque hemorrhage is seen as high-signal (arrows) in the carotid wall. B, Corresponding H&E-stained section of carotid endarterectomy specimen confirms the presence of intraplaque hemorrhage (arrows).
Special Procedures
Angiography is considered the reference standard against which noninvasive modalities are compared. Angiography assesses the vessel’s luminal diameter and is dependent on a view tangential to the tightest stenosis. In the presence of the typical eccentric stenosis two projections are insufficient to achieve this goal. It is unsurprising, therefore, that compared with 3D DSA, conventional DSA is repeatedly shown to underestimate luminal stenosis.49,73 In the absence of calibration methods, expression of stenosis severity is limited to a percent stenosis relative to the distal ICA. The NASCET measure has been adopted as the technique of choice. The technique of stenosis measurement is well described.74 The tightest luminal diameter at the stenosis site is measured (a). The normal distal ICA is measured at a site where the walls are parallel (b). The percentage stenosis is calculated by the equation 1 − (a/b). Assessment of plaque morphology including ulceration is poor, with a reported sensitivity of 46%. Nonetheless, angiography is very sensitive to calcifications and/or luminal thrombosis.28 Limitations include the risk of permanent or transient neurologic deficit (0.5%-0.9%, respectively) use of ionizing radiation, and use of iodinated contrast media.
Angiography may underestimate stenosis where diffuse atherosclersis narrows the entire arterial lumen and in early atherosclerosis where outward displacement of the vessel wall occurs.24 The measurement of near occlusion will underestimate stenosis using the NASCET ratio. For this reason and those outlined earlier, the exclusion of near occlusion is important. Angiographic features include ipsilateral carotid artery smaller than contralateral ICA, smaller or equal to ipsilateral external carotid artery (ECA), and delayed filling relative to the ipsilateral superficial temporal artery.
A variety of radiopharmaceuticals have been designed to image atherosclerotic plaques. These include labeling of lipoproteins (e.g., low-density lipoprotein [LDL]), as well as antibodies against cells (macrophages) or cell surface proteins (e.g., intercellular adhesion molecule-1 [ICAM-1]) with agents such as technetium (99mTc) and indium (111In, 123In). Experimental models of atherosclerosis have shown promising results, but the technique is limited by poor signal-to-noise ratios and prolonged clearance of the radiopharmaceuticals.75
Fluorodeoxyglucose-labeled positron emission tomography (FDG-PET) is a promising technique. There is a fast clearance rate and better contrast-to-noise compared with other radiotracers.76 Macrophages have high FDG uptake and are shown to be good markers of plaque inflammation. FDG thus has good potential to evaluate macrophage density in plaques.77,78
Treatment
Treatment of Carotid Disease
Carotid endarterectomy (CEA) is the primary treatment for atherosclerotic carotid artery disease, so the use of this surgical technique has steadily increased during the past decades.79 Its increased use is in part due to many randomized, controlled clinical trials where both symptomatic and asymptomatic carotid stenosis were studied. The two major trials assessing benefit from carotid endarterectomy in patients with symptomatic carotid artery stenosis are the NASCET80 and the European Carotid Surgery Trial (ECST).81
For patients with symptomatic moderate carotid stenosis (50%-69%), the benefits are less than for high-grade stenosis. NASCET demonstrated some benefit, but no significant benefit was seen in ECST. Patients with low-grade stenosis (<50% stenosis) did not benefit in either study.
The 2011 U.S. guidelines endorsed by multiple societies, including the American Heart Association (AHA), recommend carotid endarterectomy for patients with symptomatic stenosis between 70% and 99% within 6 months of a TIA or ischemic stroke provided that the periprocedural morbidity and mortality is lower than 6%. They recommend that carotid endarterectomy should be considered in moderate carotid artery stenosis, depending on associated comorbidities, but not in patients with low-grade carotid artery stenosis.82–84a
The presence of asymptomatic carotid artery stenosis is a risk factor for strokes, but the risk is lower when compared with that of symptomatic carotid artery stenosis.12 The Asymptomatic Carotid Atherosclerosis Study (ACAS) is the largest randomized controlled trial evaluating the effects of carotid endarterectomy on asymptomatic patients with carotid artery stenosis.85 In this trial, patients with asymptomatic 60% to 99% carotid stenosis were randomized to receive either medical treatment or carotid endarterectomy. The latter was found to be more beneficial than medical treatment; however, the ACAS reported a smaller absolute risk reduction when compared with the trials in symptomatic patients.
Benefit from carotid endarterectomy is greatest when the presentation is stroke, followed by TIA and retinal events. There is also a trend to greater benefit in patients with irregular plaques.86 Men have a greater benefit from carotid endarterectomy with lower rates of perioperative complications compared with women. The indications of carotid endarterectomy for asymptomatic patients with carotid artery stenosis are less clear. The guidelines from the American Heart Association and the National Stroke Association (U.S.) recommend that carotid endarterectomy should be considered in patients younger than 80 years old with asymptomatic carotid stenosis greater than 60%. Surgery should be offered by experienced surgeons with low complication rates. Patient comorbidities, life expectancy, and patient preference should also be considered.82–84
Carotid Angioplasty and Stenting
Endovascular treatment of carotid stenosis is less invasive than carotid endarterectomy. Stent deployment is favored over angioplasty alone. Advantages of endovascular treatment include ability to perform the procedure under local rather than general anesthesia. Surgery requires a neck dissection, which increases the risk of cranial nerve injury and wound sepsis. Complications related to carotid artery stenting (CAS) are secondary to periprocedural ischemic events, arterial puncture, and iodinated contrast administration. Periprocedural TIAs and strokes are largely the result of atheroembolus at the time of angioplasty or stenting. Filter devices, occlusion balloons, and basket devices have been developed in an attempt to minimize these occurrences. These devices appear to work. A systematic review of the literature reported combined stroke and death rate for symptomatic and asymptomatic patients of 1.8% versus 5.5% with and without cerebral protection devices, respectively. Minor stroke accounted for the majority of events (3.7% vs. 0.5% with protection).87 Stroke reduction with cerebral protection devices is indeed confirmed in the recent, prematurely terminated study. A protection device was utilized in 78% and 27% of patients in the EVA-3S and SPACE trials, respectively. SPACE found no difference in the primary outcome measures in patients with and without cerebral protection devices. A stroke reduction was reported in EVA-3S. However, the magnitude of reduction was not sufficient to demonstrate any benefit of carotid artery surgery over carotid endarterectomy. Furthermore, diffusion-weighted imaging (DWI) studies performed after stenting have shown significant difference in the incidence of silent infarcts in patients with and without cerebral protection. The clinical significance of these subclinical infarcts, however, is not known.
Studies of both carotid angioplasty and stenting have demonstrated varying results. Earlier studies, including CAVATAS,88 Wallstent, SAPPHIRE,89 SPACE,90 and EVA-3S,91 have failed to demonstrate benefit over carotid endarterectomy. CREST, the most recent and largest study to date, has provided support for carotid stenting, showing similar long-term outcome between CAS and CEA in symptomatic and asymptomatic patients.91a Differences in periprocedural (<30 days) complications remain the major focus of current studies and are a leading consideration in selection of the most appropriate procedure. The Carotid Stent Trialists Collaboration (CSTC) meta-analyzed data from EVA-3S, SPACE, and ICSS (3433 patients) and showed that the 30-day rate of stroke or death was significantly higher after CAS (7.7%) versus CEA (4.4%); risk ratio for CAS relative to CEA 1.74 (1.32 to 2.30, p = 0.0001)91b The CREST trial showed similar findings, including higher periprocedural (30-day) stroke rate for CAS (4.1%) versus 2.3% for CEA (p = 0.01). Conversely, periprocedural myocardial infarctions were higher for CEA (2.3%) than for CAS (1.1%, p = 0.03) in the CREST study. Another important observation from CREST was that CAS provided slightly better outcomes in younger (<70-year-old) patients compared to CEA. In conclusion, there is increasing evidence to support the use of carotid stenting. Selected patients with age younger than 70; cardiac comorbidities, including congestive heart failure, recent unstable angina, or myocardial infarction; surgically inaccessible lesions; prior endarterectomy; or neck irradiation may benefit from endovascular treatment.
INTRACRANIAL ATHEROSCLEROSIS
Epidemiology
Intracranial atherosclerosis accounts for 8% to 12% of all ischemic strokes,92 corresponding to 70,000 cases per year in the United States.93 Much attention has been paid to the extracranial carotid arteries as a source of ischemic cerebrovascular disease since Fisher’s 1951 report. Furthermore, NASCET has emphasized measurements of extracranial carotid stenosis to stratify patients into treatment regimens. Disease in the intracranial circulation was often attributed to spread from more proximal disease, such as emboli. However, in the Joint Study of Extracranial Arterial Occlusion, 6.1% of patients had isolated intracranial disease, which was independently and highly associated with stroke.94
The incidence varies widely among populations. Asians, blacks, and Hispanics have a much higher incidence of intracranial atherosclerosis than whites. Conversely, whites have a much higher incidence of extracranial carotid atherosclerosis. In China, intracranial disease accounts for one third of ischemic strokes.92 The reason for this variation is not yet known. In the Northern Manhattan Stroke Study intracranial atherosclerosis was found in 11% of Hispanic, 6% of black, and 1% of white stroke patients.95 The stroke risk factors of diabetes and hypercholesterolemia were also determinants of intracranial atherosclerotic disease. In the United States there is a fourfold higher incidence of MCA disease in Chinese-American stroke patients than whites.96 Conversely, whites with strokes had a fivefold higher incidence of severe extracranial carotid disease. Pathologic data from deceased Hong Kong Chinese97 showed a 31% incidence of atherosclerosis in the intracranial arteries. In symptomatic patients enrolled in the Extracranial/Intracranial Bypass Study, 58% of Asians, 34% of blacks, and only 18% of whites had MCA disease.98
Older age, hypertension, ischemic heart disease, hypercholesterolemia, cigarette smoking, and diabetes are factors associated with MCA stenosis. Among asymptomatic Asian patients with vascular risk factors (hypertension, diabetes, or hyperlipidemia) the prevalence of MCA stenosis increases with the number of risk factors present (7.2% for one, 10.6% for two, 20.4% for three, 29.6% for four, and 12.6% overall incidence for any risk factors).99 Vascular risk factors alone do not entirely explain MCA stenosis, because Western whites have similar risk factors but a much lower incidence of disease. Interestingly, obesity (as measured by body mass index) is inversely associated with MCA stenosis. The reason is uncertain but does correspond to a Japanese study that found low body mass index (<18) was associated with a higher risk of total stroke and intraparenchymal hemorrhage.100
Atherosclerosis is a systemic process, with disease in one vascular system predictive that others may also be affected. The presence of plaques in the aorta is associated with coronary artery disease and thickening of the extracranial carotid artery. Aortic plaques are also an independent predictor of intracranial atherosclerotic disease.101
Clinical Presentation
Patients with intracranial atherosclerotic disease are at high risk for stroke. Stroke incidence varies with degree of stenosis and presence of symptoms. Patients with at least 30% intracranial stenosis have an annual ipsilateral stroke and death rate of 3.1% to 8.1% and 4.7% to 17.2%, respectively.102,103 Highest stroke rates occur with MCA occlusion.92 Symptomatic patients with more than 50% stenosis had a recurrent stroke rate of 32% over a 6-month period92 whereas 61% of those with more than one stenotic artery had a recurrent stroke in the same period. The Standford Stroke Centre study reported an annual stroke or death rate of 45% in patients with intracranial stenosis failing medical therapy. These mostly occurred within the first 36 days.104 The Groupe d’tude des Stenoses Intra-Craniennes Atheromateuses Symptomatiques (GESICA) study assessed the natural history of symptomatic patients on medical treatment with 50% to 99% stenosis.105 After a 23-month follow-up, 38.2% had either an ipsilateral stroke (13.7%) or TIA (24.5%), with a median of 2 months between the initial and recurrent event. Those with hemodynamically significant stenosis had a 60.7% incidence of a recurrent stroke or TIA. Stenoses were classified as hemodynamically significant if symptoms related to the stenosis occurred during a change in position, on effort, or if there was an introduction or increase of an antihypertensive medication.
The risk of stroke associated with posterior circulation intracranial disease (vertebral, basilar, posterior cerebral artery [PCA], or posterior inferior cerebellar artery [PICA]) is 2.5% to 5.5% per year.106 As a subset of the Warfarin-Aspirin Symptomatic Intracranial Disease trial,107 22% of patients with symptomatic posterior circulation disease greater than 50% had an ipsilateral stroke after 13.8 months. Patients with basilar or vertebral artery disease and greater degrees of stenosis had the highest stroke rates.106 Patients with more than 50% stenosis of the distal vertebral artery or basilar artery had an 18% incidence of stroke over 6.1 years (11.4% in the same territory as the stenotic lesion). Distal vertebral disease had a higher stroke or TIA rate than proximal disease.
Pathophysiology
Atherosclerotic disease is a dynamic process that can progress, regress, or remain stable over time. Serial angiographic follow-up of intracranial disease has shown that up to 80% remain stable or progress over 26.7 months while the remaining 20% regress.102 The ICA is the most frequent site of intracranial stenosis (50% of cases).102 The morphology of the plaque is important in determining plaque stability. Plaque morphology can be either white or yellow.103 White plaques have thicker fibrous caps and lower distensibility, giving them more stability. Yellow plaques are often found in acute coronary syndromes and tend to have a thinner fibrous cap, lipid-rich core, and higher distensibility, resulting in more vulnerability and instability. As yellow plaques heal over time, they tend to become white.
The presence of atherosclerotic disease in the intracranial arteries can lead to stroke via one of three mechanisms: (1) reduced perfusion; (2) thromboembolism, and (3) penetrating artery occlusion.103 The first two are similar to the pathophysiology of extracranial carotid disease whereas the third is unique to the intracranial circulation. Perfusion failure occurs when the stenosis causes a reduction in flow that cannot be compensated by collateral circulation. The brain maintains metabolism by reflex autodilatation and increased oxygen extraction. When both mechanisms reach their limit, a further decrease in perfusion leads to cell death. The subgroup of patients with perfusion failure may benefit from endovascular therapy. Intracranial atherosclerosis can also lead to and occurs when there is occlusion of the origin of small penetrating arteries resulting in strokes in the deep structures or brain stem.
Imaging
Ultrasonography
Transcranial Doppler (TCD) is a noninvasive and portable modality for assessing the intracranial vessels. A 2-MHz transducer is typically used through the temporal window with the patient supine. A depth of 50 to 60 mm is typically used for the MCA. The main application of TCD is in velocity measurements. The greater the degree of stenosis, the higher the velocity of blood flow through the narrowing. There have been various studies attempting to correlate measured velocities with degree of stenosis. One study comparing transcranial Doppler imaging to MRA found an optimal PSV cutoff of 140 cm/s defined MCA stenosis of more than 50% with a sensitivity of 91.4% and specificity of 82.7%. The optimal cutoff for stenosis greater than 75% was 180 cm/s.108
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree