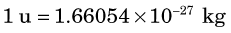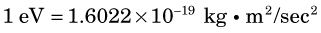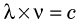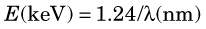chapter 2 Basic Atomic and Nuclear Physics
Radioactivity is a process involving events in individual atoms and nuclei. Before discussing radioactivity, therefore, it is worthwhile to review some of the basic concepts of atomic and nuclear physics.
a Quantities and Units
2 Mass and Energy Units
The basic unit of mass is the unified atomic mass unit, abbreviated u. One u is defined as being equal to exactly 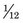 the mass of an unbound 12C atom* at rest and in its ground state. The conversion from SI mass units to unified atomic mass units is1
the mass of an unbound 12C atom* at rest and in its ground state. The conversion from SI mass units to unified atomic mass units is1
The universal mass unit often is called a Dalton (Da) when expressing the masses of large biomolecules. The units are equivalent (i.e., 1 Da = 1 u). Either unit is convenient for expressing atomic or molecular masses, because a hydrogen atom has a mass of approximately 1 u or 1 Da.
Relationships between various units of mass and energy are summarized in Appendix A. Universal mass units and electron volts are very small, yet, as we shall see, they are quite appropriate to the atomic scale.
b Radiation
Radioactive decay processes, discussed in Chapter 3, result in the emission of radiation in both of these forms.
where c is the velocity of light.
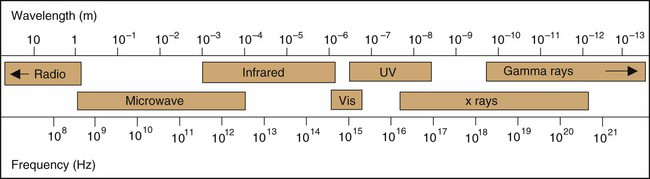
Figure 2-1 illustrates the photon energies for different regions of the electromagnetic spectrum. Note that x rays and γ rays occupy the highest-energy, shortest-wavelength end of the spectrum; x-ray and γ-ray photons have energies in the keV-MeV range, whereas visible light photons, for example, have energies of only a few electron volts. As a consequence of their high energies and short wavelengths, x rays and γ rays interact with matter quite differently from other, more familiar types of electromagnetic radiation. These interactions are discussed in detail in Chapter 6.
c Atoms
1 Composition and Structure
Events of the late 19th and early 20th centuries, beginning with the discovery of x rays by Roentgen (1895) and radioactivity by Becquerel (1896), revealed that atoms had a substructure of their own. In 1910, Rutherford presented experimental evidence indicating that atoms consisted of a massive, compact, positively charged core, or nucleus, surrounded by a diffuse cloud of relatively light, negatively charged electrons. This model came to be known as the nuclear atom. The number of positive charges in the nucleus is called the atomic number of the nucleus (Z). In the electrically neutral atom, the number of orbital electrons is sufficient to balance exactly the number of positive charges, Z, in the nucleus. The chemical properties of an atom are determined by orbital electrons; therefore the atomic number Z determines the chemical element to which the atom belongs. A listing of chemical elements and their atomic numbers is given in Appendix B.
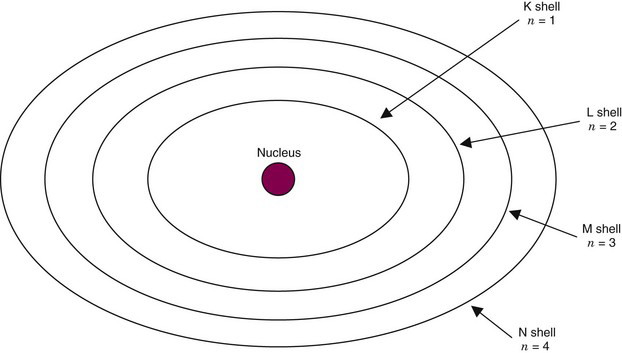
Each shell actually comprises a set of orbits, called substates, which differ slightly from one another. Each shell has 2n − 1 substates, in which n is the quantum number of the shell. Thus the K shell has only one substate; the L shell has three substates, labeled LI, LII, LIII; and so forth. Figure 2-2 is a schematic representation of the K, L, M, and N shells of an atom.
2 Electron Binding Energies and Energy Levels
In the most stable configuration, orbital electrons occupy the innermost shells of an atom, where they are most “tightly bound” to the nucleus. For example, in carbon, which has a total of six electrons, two electrons (the maximum number allowed) occupy the K shell, and the four remaining electrons are found in the L shell. Electrons can be moved to higher shells or completely removed from the atom, but doing so requires an energy input to overcome the forces of attraction that “bind” the electron to the nucleus. The energy may be provided, for example, by a particle or a photon striking the atom.
The energy required to completely remove an electron from a given shell in an atom is called the binding energy of that shell. It is symbolized by the notation KB for the K shell,* LB for the L shell (LIB, LIIB, LIIIB for the L shell substates), and so forth. Binding energy is greatest for the innermost shell, that is, KB > LB > MB. Binding energy also increases with the positive charge (atomic number Z) of the nucleus, because a greater positive charge exerts a greater force of attraction on an electron. Therefore binding energies are greatest for the heaviest elements. Values of K-shell binding energies for the elements are listed in Appendix B.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree