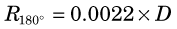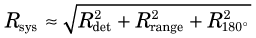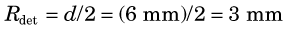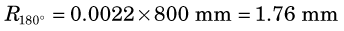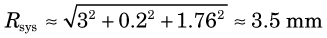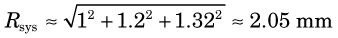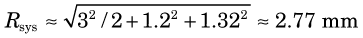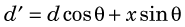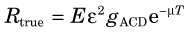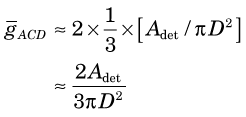chapter 18 Positron Emission Tomography
The second major method for tomographic imaging in nuclear medicine is positron emission tomography (PET). This mode can be used only with positron-emitting radionuclides (see Chapter 3, Section G). PET detectors detect the “back-to-back” annihilation photons that are produced when a positron interacts with an ordinary electron. Although the annihilation photons could be detected using single photon emission computed tomography (SPECT) systems operating in conventional single-photon counting mode, these systems are not optimally designed for the relatively high energy of annihilation photons (511 keV). They have relatively low detection efficiencies at these energies and require relatively inefficient high-energy collimators. As well, SPECT systems do not take advantage of the back-to-back directional characteristics of annihilation photons. This unique feature is exploited advantageously with special annihilation-coincidence detector systems for PET.
A Basic Principles of Pet Imaging
1 Annihilation Coincidence Detection
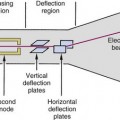
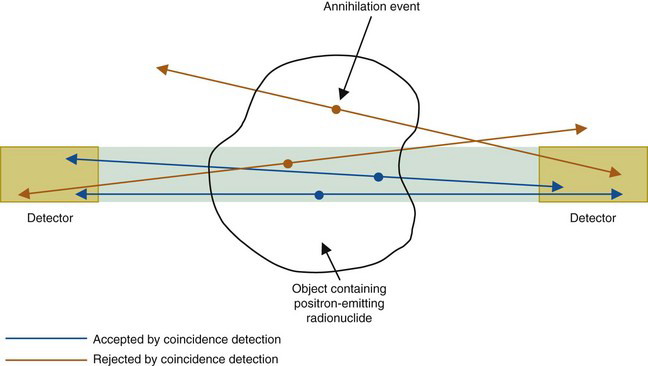
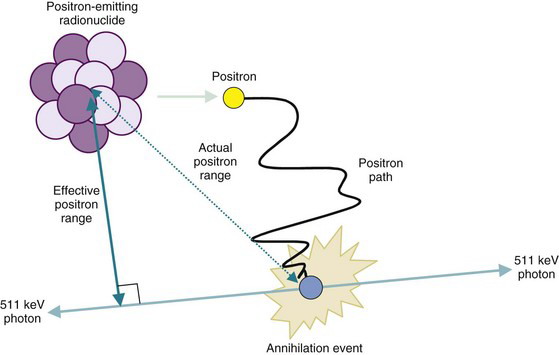
When a positron undergoes mutual annihilation with a negative electron, their rest masses are converted into a pair of annihilation photons (see Fig. 3-7). The photons have identical energies (511 keV) and are emitted simultaneously, in 180-degree opposing directions, usually within a few tenths of a mm to a few mm of the location where the positron was emitted, depending on the energy and range of the positrons. Near-simultaneous detection of the two annihilation photons allows PET to localize their origin along a line between the two detectors, without the use of absorptive collimators. This mechanism is called annihilation coincidence detection (ACD). Detection of a pair of annihilation photons in opposing detectors actually defines the volume from which they were emitted. Most ACD detectors have square or rectangular cross sections. Thus the volume is essentially a box of square or rectangular cross section, with dimensions equal to those of the detectors (Fig. 18-1).
Coincidence logic (Chapter 8, Section F and Fig. 8-15) is employed to analyze the signals from the opposing detectors. For many PET scanners, this is accomplished by having the electronics attach a digital “time stamp” to the record for each detected event. Typically, this is done with a precision of approximately 1 or 2 nanoseconds (1 nsec = 10−9 sec). The coincidence processor examines the time stamp for each event in comparison with events recorded in the opposing detectors. A coincidence event is assumed to have occurred when a pair of events are recorded within a specified coincidence timing window, which typically is 6 to 12 nanoseconds.
Although annihilation photons are emitted simultaneously, a small but finite coincidence window width is needed to allow for differences in signal transit times through the cables and electronics, as well as different distances of travel by the two photons from the annihilation event to the detectors (see Section A.2). In addition, the detectors in a PET scanner do not have perfect timing precision and therefore have a finite timing resolution. Uncertainties that govern the timing resolution can arise from the statistical nature of the signal (which is produced by the conversion of 511-keV photons into light, electrons, or electron-hole pairs in the detector) and from electronic noise in the detector and associated circuits. Uncertainties also can arise from the electronic method used to determine the time at which the interaction occurred (see Chapter 8, Section F). For a pair of similar detectors, the timing uncertainties typically are well described by a gaussian distribution, and the timing resolution is defined as the full width at half maximum (FWHM) of this distribution. For scintillation detectors, the timing uncertainty is reduced, and the timing resolution improved, by using brighter and faster scintillators that produce a large number of light photons over a short time interval immediately after an interaction occurs. Timing resolution is typically in the range of 0.5 to 5 nsec, depending on which scintillator and photodetector is used.
The need for a finite window width permits other types of events to occur in coincidence, as discussed in Section A.9. Also, as discussed in Section A.4, the annihilation photons are not always emitted in precise back-to-back directions. The effects of these deviations from the ideal are discussed in the sections indicated.
The ability of ACD to localize events on the basis of coincidence timing, without the need for absorptive collimation, is referred to as electronic collimation. As was discussed in Chapter 14, Section C, the lead septa in standard parallel-hole collimators, which are necessary to obtain adequate spatial localization, also are responsible for the relatively low sensitivity of these collimators. Because ACD does not require a collimator to define spatial location, its sensitivity (number of events detected per unit of activity in the object) is much higher than is obtainable with the absorptive collimators used for conventional planar imaging and for SPECT. For comparable midplane resolution, the sensitivity of PET is many times higher than for SPECT.
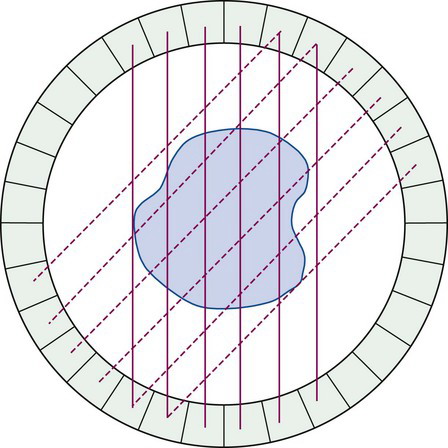
In addition, by incorporating multiple opposing detectors in a complete ring or other geometric array around the patient, and operating each detector in the array in coincidence with multiple detectors on the other side of the array, data for multiple projection angles can be acquired simultaneously (Fig. 18-2). Indeed, with a stationary ring or geometric array that completely surrounds the patient, it is possible to acquire data for all projection angles simultaneously. This allows the performance of relatively fast dynamic studies and the reduction of artifacts caused by patient motion.
2 Time-of-Flight PET
With the fastest available scintillators and careful design of electronic components and connections, it is possible to achieve timing accuracy at the level of a few hundred picoseconds. Although this is adequate to achieve localization only to within a few centimeters, images reconstructed from data acquired at this level of timing resolution have a higher signal-to-noise ratio than images reconstructed without time-of-flight information. This is because individual events can be constrained to lie within a smaller volume in the image reconstruction process. Figure 18-3 illustrates how the backprojection of data along one particular line of response is constrained to a smaller region of the reconstructed image matrix by the addition of time-of-flight information. To provide practically useful levels of time-of-flight information, only the fastest and brightest scintillators, such as LSO and LaBr3, can be used (see Tables 7-2 and 18-2). Several commercially built systems incorporate some level of time-of-flight information using these materials. Although there are scintillators with even faster decay components, such as BaF2, these are not favored because the signal-to-noise improvements that can be realized from time-of-flight information is typically more than offset by their lower density and therefore lower efficiency for detecting 511-keV annihilation photons.
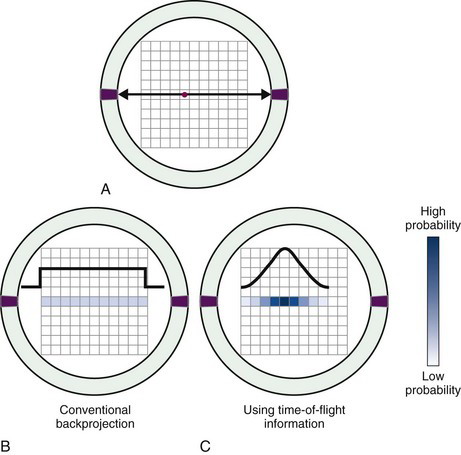
FIGURE 18-3 A, A pair of annihilation photons are emitted from a source (red dot) and detected in coincidence by opposing detectors. B, In the absence of time-of-flight information, there is no information about the location of the source along the line joining the two detectors. During reconstruction, the event is backprojected with equal probability of having occurred in all pixels along that line. C, With time-of-flight information, some limited localization of the event is possible and events are backprojected with probabilities that follow a Gaussian distribution, centered on pixel Δd (Equation 18-1) from the center of the scanner and with a full width at half maximum equal to the timing resolution of the detector pair.
3 Spatial Resolution: Detectors
The spatial resolution of ACD with discrete detector elements is determined primarily by the size of the individual detector elements. As shown in Figure 18-4A, for elements of width d, a one-dimensional (1-D) slice through the ACD point-source response profile at midplane between the detector pair is a triangle. The detector resolution, Rdet has a FWHM = d/2. As the source moves toward either detector, the response profile becomes trapezoidal, eventually becoming a box of width d at the face of either detector. Considering a 2-D detector with width d and height h, the ACD response profile becomes a 3-D function, which is a pyramid at midplane and a rectangular box of area d × h at the face of either detector. Between these extremes, it is the frustum of a pyramid with lower base size equal to the size of the detectors and upper base size increasing linearly from zero at midplane to the size of the detectors at their face.
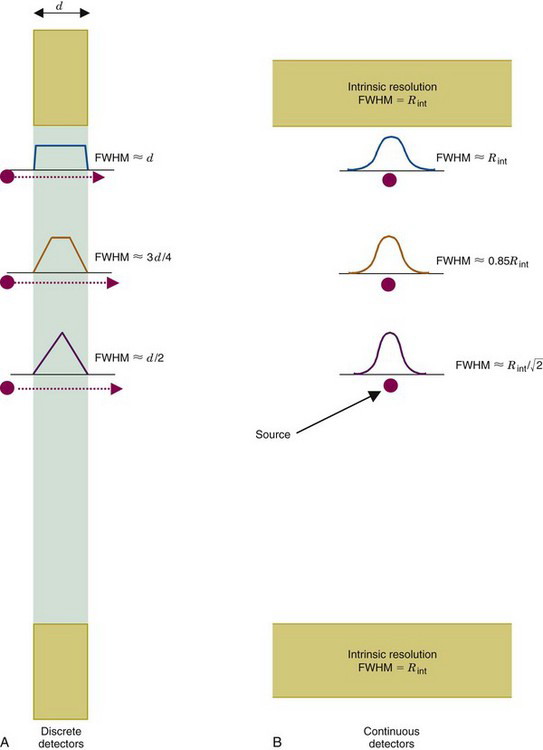
FIGURE 18-4 Spatial resolution of detector pair (Rdet) for coincidence detection. A, For discrete detectors, spatial resolution is determined by the width of the detector element, d. At midplane, the coincidence response function is a triangle with full width at half maximum (FWHM) = d/2. As the source is moved closer to one of the detectors, the response function becomes trapezoidal in shape, eventually becoming a rectangle of width, d. B, For continuous detectors, spatial resolution is determined by the intrinsic resolution of the detector, Rint. At the midplane, the coincidence response function is approximately gaussian, with FWHM = Rint /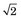 . Near the face of a detector, it becomes FWHM = Rint.
. Near the face of a detector, it becomes FWHM = Rint.
Alternatively, consider an uncollimated pair of gamma camera detectors, also operating in coincidence mode (Fig. 18-4B). If their intrinsic spatial resolution (see Chapter 14, Section A.1) is a gaussian function with FWHM = Rint, then the spatial resolution of the detector pair for ACD also is a gaussian function with FWHM = Rint /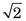 at midplane. The ACD response profile becomes wider as the source moves toward either detector, with its FWHM eventually becoming equal to Rint at the face of either detector. Assuming that the resolution of the imaging detector is the same in all directions, the 2-D ACD response profile is obtained by rotating the 1-D gaussian function around its center.
at midplane. The ACD response profile becomes wider as the source moves toward either detector, with its FWHM eventually becoming equal to Rint at the face of either detector. Assuming that the resolution of the imaging detector is the same in all directions, the 2-D ACD response profile is obtained by rotating the 1-D gaussian function around its center.
For both discrete or gamma camera–type detectors, the spatial resolution of ACD varies by only approximately 30% in the central 60% of the space between the detectors (Fig. 18-5). By comparison, the resolution of a parallel-hole collimator can vary by several hundred percent over a comparable range (see Fig. 14-19). When profiles obtained from opposing views in SPECT are combined using the geometric mean, the variation in resolution within the space between the opposing detectors is reduced to a level comparable to ACD (see Fig. 17-8). The key difference is that the resolution of ACD is determined primarily by the size of the detector element or the intrinsic resolution of the camera detector, whereas for SPECT, the resolution is primarily determined by the collimator resolution at the midpoint between the two detectors. The latter is substantially degraded from its value at the face of the detector. This means that the collimator must have very high resolution at its face to achieve even moderately good resolution at midplane between the detectors. In turn, the requirement for high spatial resolution leads to relatively low detection efficiency with absorptive collimators (see Chapter 14, Section C). As discussed in Section A.8, this results in relatively low sensitivity for a SPECT system as compared with a PET system with comparable spatial resolution.
4 Spatial Resolution: Positron Physics
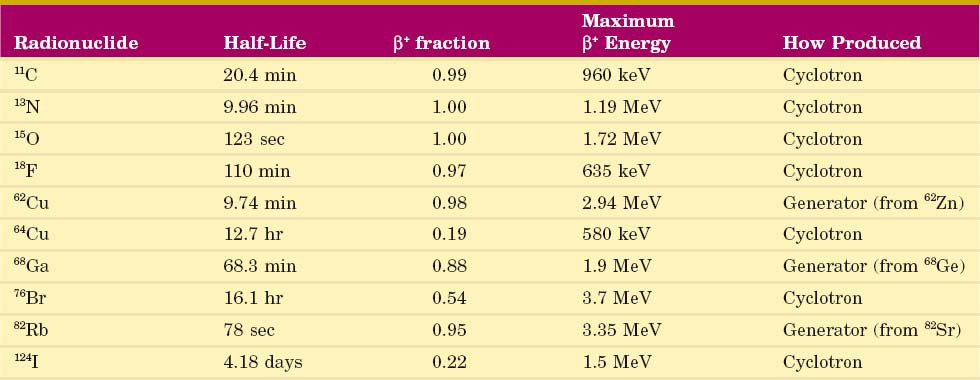
The spatial resolution of an ACD system is degraded from the values derived from simple geometry indicated in Figure 18-4 by two factors relating to the basic physics of positron emission and annihilation. The first is the finite range of positron travel before it undergoes annihilation. ACD defines the line along which the annihilation event took place, which is not precisely the location from which the decaying radioactive nucleus emitted the positron. The range of travel for a positron before it undergoes annihilation is essentially the same as the range of travel of an ordinary electron (or β particle) of similar energy (see Chapter 6, Section B.2). Figure 6-10 and Table 6-1 show the extrapolated range versus maximum energy for β particles, 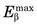 . The maximum energies of the positrons emitted from radionuclides used for nuclear medicine are in the range of 0.5 to 5 MeV (Table 18-1; see also Appendix C). Thus their extrapolated ranges are in the 0.1- to 2-cm range.
. The maximum energies of the positrons emitted from radionuclides used for nuclear medicine are in the range of 0.5 to 5 MeV (Table 18-1; see also Appendix C). Thus their extrapolated ranges are in the 0.1- to 2-cm range.
The extrapolated range applies to the highest-energy positrons emitted by a radionuclide. However, positrons, like β particles, are emitted with a spectrum of energies. Only a small fraction have the full amount of energy available from the decay (see Fig. 3-2). In addition, the extrapolated range is the maximum distance that the electron would travel if it were not significantly deflected in any of its interactions and traveled in essentially a straight line to the end of its range. In reality, most electrons (and positrons) travel a tortuous path, often with multiple large-angle deflections (see Fig. 6-4). The result is that the average distance measured from the origin of the positrons to the end of their path is significantly smaller than their extrapolated range.
For purposes of defining the spatial resolution of ACD, the distance of interest is the effective positron range. This is the average distance from the emitting nucleus to the end of the positron range, measured perpendicular to a line defined by the direction of the annihilation photons (Fig. 18-6). This distance always is smaller than the extrapolated range for the positrons emitted by the radionuclide.
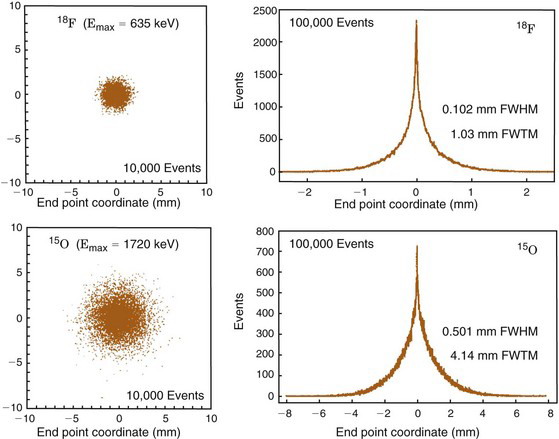
Figure 18-7 shows the positron range distribution for point sources of 18F (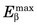 = 0.635 MeV) and 15O (
= 0.635 MeV) and 15O (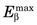 = 1.72 MeV). According to Figure 6-10, the extrapolated ranges for these positrons in water would be approximately 2 mm and 8 mm, respectively; however, the FWHMs of their distribution profiles are only 0.1 mm and 0.5 mm.
= 1.72 MeV). According to Figure 6-10, the extrapolated ranges for these positrons in water would be approximately 2 mm and 8 mm, respectively; however, the FWHMs of their distribution profiles are only 0.1 mm and 0.5 mm.
Note as well that the positron range distributions shown in Figure 18-7 have long tails and thus are not well described by gaussian functions. Therefore the FWHM is not the best indicator of the effect of positron range on ACD spatial resolution. Instead, the root mean square (rms) effective range often is used. Figure 18-8 shows the general relationship between rms effective range and maximum positron energy. Typical rms effective ranges (and thus the blurring caused by positron ranges) are on the order of 0.5 to 3 mm. Note that positron range is inversely proportional to the density of the absorber. Thus rms ranges would be proportionately higher in lung tissue and airways (ρ ~ 0.1-0.5 g/cm3) and lower in dense tissues such as bone (ρ ~ 1.3-2 g/cm3).
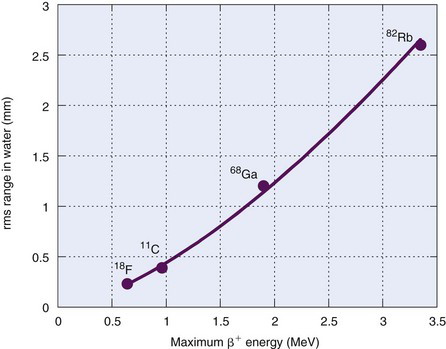
FIGURE 18-8 Root mean square range for positrons in water versus 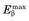 .
.
(Data from Derenzo SE: Mathematical removal of positron range blurring in high-resolution tomography. IEEE Trans Nucl Sci 33:565-569, 1986.)
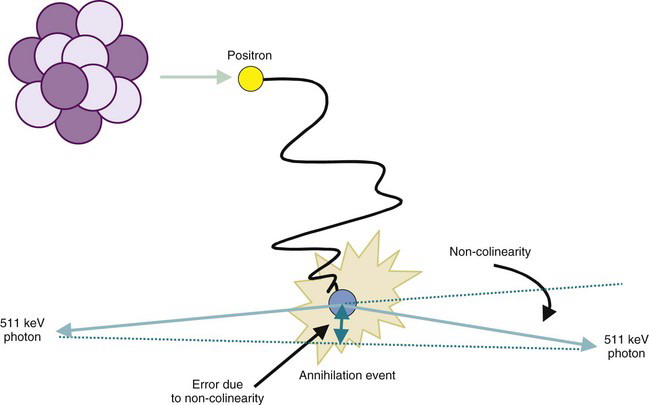
A second factor involving the physics of positrons is that the annihilation photons almost never are emitted at exactly 180-degree directions from each other (Fig. 18-9). This effect, which is due to small residual momentum of the positron when it reaches the end of its range, is known as noncolinearity. The angular distribution is approximately gaussian with FWHM approximately 0.5 degree. The effect on spatial resolution, expressed in terms of FWHM, is linearly dependent on the separation of the ACD detectors, D, and is given by
The system resolution of an ACD or PET detector system is obtained by combining the individual resolution components, in the same manner as the component resolutions are combined to determine the system resolution for a gamma camera system (see Chapter 14, Section C.4). Thus
where Rdet is the spatial resolution of the detector system, as determined by the size of the discrete detector elements or the intrinsic resolution of continuous detectors (see Section A.3 and Fig. 18-4). For typical whole-body PET scanners, with either discrete detector elements or gamma camera detectors, the effects of positron range and noncolinearity combine to add anywhere from a few tenths of a millimeter to a few millimeters to system resolution.
Answer
The rms range for 18F is Rrange ≈ 0.2 mm (see Fig. 18-8), and the noncolinearity for an 80-cm separation is
Using Equation 18-3, the system resolution is
For situation 2, using the same equations and Figure 18-8, the spatial resolution of 2-mm-wide discrete detectors is Rdet = 1 mm, the rms range for 68Ga is Rrange ≈ 1.2 mm and the noncolinearity blurring for the 60-cm separation is 1.32 mm. Thus the system resolution is
For situation 3, Rdet = 3 /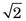 ≈ 2.12 mm (see Fig. 18-4) while other factors remain the same as in situation 2. Thus
≈ 2.12 mm (see Fig. 18-4) while other factors remain the same as in situation 2. Thus
From Example 18-1, one can conclude that positron range and noncolinearity have a relatively small effect on system resolution for whole-body systems, which usually have larger detector elements (situation 1) or only moderately high spatial resolution (situation 3). This is especially true for 18F, the radionuclide most commonly used for clinical applications. However, their effects can be important limitations for high-resolution brain imaging or small-animal imaging devices (see Section B.5) that employ small discrete detector elements, especially for applications involving higher-energy positrons (situation 2).
5 Spatial Resolution: Depth-of-Interaction Effect
A substantial thickness of scintillator material is required to efficiently stop 511-keV annihilation photons. In a gamma camera, typical NaI(Tl) detector crystal thicknesses are 1.25 cm or less. PET systems generally employ 2- to 3-cm-thick scintillators with greater stopping power, such as BGO or LSO. For PET systems using arrays of detectors in multiple coincidence mode around the object, the relatively thick detector elements lead to a degradation of resolution known as the depth of interaction (DOI) effect. Although the effect also occurs in the axial direction in scanners that use cross-plane coincidence detection for 3-D data acquisition (see Section C.2), the primary effect is in the radial direction, and the discussion focuses on this aspect of the problem.
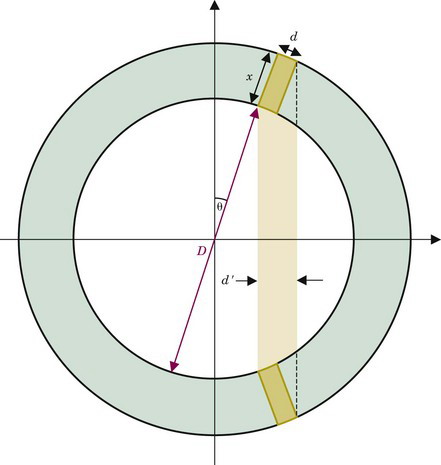
Figure 18-10 illustrates the cause of the problem for a detector system that uses a circular array of elements, all or most elements of which operate in multicoincidence mode with other elements in the array. For a source located near the center of the scanner, spatial resolution is determined by the width of the detector element, Rdet = d/2, as described in Section A.3 and illustrated in Figure 18-4. However, for a source located away from the center, the apparent width of the detector element becomes
where d, x, and θ are as indicated in Figure 18-10. The apparent change in width results from the angulation between the detectors and from lack of knowledge about the depth at which an interaction has occurred within the detector crystal. The spatial resolution (FWHM) then becomes R′det = d′/2. Using Equation 18-4, this can be written as
Equation 18-5 is only an approximation because the thickness of detector material is not constant across the width of the detector element seen by the source. Note as well that, for thin detector elements [(x/d) << 1], it is possible that R′det < Rdet. The same would be true for a very efficient detector material (or a very thin detector) that would stop most of the annihilation photons in a thin layer at the entrance to the detector. However, these conditions never apply in practice. Typically, x ~ 2 to 3 cm and d ~ 0.3 to 0.6 cm. For a whole-body PET scanner with 4-mm-wide detectors on a diameter of 80 cm, the DOI effect causes approximately a 40% degradation of resolution at a distance of 10 cm from the center of the field of view (FOV).
Note also in Equations 18-4 and 18-5 that, for a given radial distance away from the center of the scanner, θ and sin θ become smaller (and thus R′det becomes smaller) as the diameter of the detector ring becomes larger. Because of the DOI effect, PET scanners often are built with detector arrays that are of larger diameter than would be necessary to fit the patient, which in turn increases detector costs.
6 Spatial Resolution: Sampling
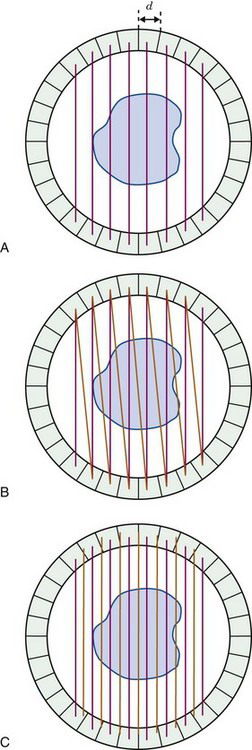
In ACD, the FWHM of the detector resolution is one-half the width of a detector element (see Fig. 18-4). For a stationary array of detector elements, each of width d, the sampling interval between parallel projection lines also is d (Fig. 18-11A). Considering only detector resolution, it can be shown that this leads to undersampling of object profiles, which in turn leads to a distortion of the high-frequency content (i.e., the fine details) of the reconstructed image (see Fig. 16-11 and Section C). According to Equation 16-14, three samples should be acquired per FWHM of spatial resolution. In theory, this would translate into a requirement for six samples over an interval equal to the width of a detector element. However, as described elsewhere in this section, system resolution is degraded from the theoretical limits established by intrinsic resolution by other factors, so that coarser sampling is acceptable. Nonetheless, some additional sampling is required beyond that illustrated in Figure 18-11A.
In practice, two samples usually are acquired per detector element width. Although less than the theoretical ideal, this results in little noticeable distortion of clinical images. Some earlier scanners actually incorporated a mechanical shift to acquire two sets of data with the detector elements shifted by half the width of a detector element between acquisitions. This approach was mechanically cumbersome. The modern approach is to use coincidence events in adjacent pairs of detector elements, thereby creating additional samples between the detector elements, as illustrated in Figure 18-11B. These are treated as samples acquired with a virtual set of detectors located between the actual detector elements, as shown in Figure 18-11C. The ray paths for the additional samples are not quite parallel to those for directly opposed detectors, but this seems to have little effect on the reconstructed image across most of the useful FOV.
Note that combining data for adjacent pairs of detectors into a single projection view as illustrated in Figure 18-11B and C, reduces the number of projection angles available from a stationary ring of detectors by half. Note also that PET systems that use continuous (i.e., gamma camera type) detectors (see Section B.3) can use arbitrarily chosen sampling intervals and thus can avoid some of the sampling problems associated with discrete detector arrays.
7 Spatial Resolution: Reconstruction Filters
The discussions in the preceding sections describe the spatial resolution achievable with PET systems, as determined by the physical characteristics of the imaging device and the basic physics of positron decay. However, as discussed in Chapter 16, Sections B.3 and C.3, spatial filters are applied to the recorded projection profiles to suppress noise in the reconstructed image (see Fig. 16-12). Inevitably, this results in some degradation of spatial resolution. In general, the fewer the number of counts recorded in an image, the lower the filter cut-off frequency (kcut-off) and the greater the loss of spatial resolution.
The selection of cut-off frequency depends in part on the type of study. Thus a brain scan might be reconstructed with a cut-off frequency yielding 6-mm spatial resolution, whereas an abdominal scan, with more tissue attenuation and generally lower count densities, might be reconstructed with a filter yielding 10-mm spatial resolution. As well, the sensitivity (number of counts recorded per unit of activity in the patient) affects the statistical quality of the image and the degree of spatial filtering required to achieve an acceptable noise level in the image. As discussed in the following section and in Section B, PET systems, especially those employing multiple detector rings and multi-ring coincidence detection, have substantially higher detection efficiencies (by orders of magnitude) than is achievable with typical SPECT systems. Thus PET images usually can be reconstructed with higher cut-off frequencies, and their final spatial resolution generally is superior to SPECT images.
8 Sensitivity
where E is the source emission rate (positrons/sec); ε is the intrinsic efficiency of each detector, that is, the fraction of incident photons detected (Equation 11-9, assumed to be the same for both detectors); and µ and T are the linear attenuation coefficient and total thickness of the object, respectively. gACD is the geometric efficiency of the detector pair, that is, the fraction of annihilation events in which both photons are emitted in a direction to be intercepted by the detectors.
As shown in Figure 18-4, the shape as well as the amplitude of the point-source response profile (i.e., gACD for a point source) varies with the location of the source between the two detectors. In one dimension, the profile is triangular at the midpoint between the detectors, rectangular at the face of either detector, and trapezoidal at locations between. In two dimensions, the ACD response at the midpoint is a pyramid, whereas at the surface of either of the detectors it is a rectangular box. At points between, it is the frustum of a pyramid. In all cases, the lower base is equal to the area of the detectors.
Maximum geometric efficiency for ACD is obtained for a point source located precisely at the midpoint of the centerline between the two detectors. However, as illustrated in Figure 18-4, this value does not apply when the point source is moved even slightly away from the centerline, or from the midpoint of that line. Thus a more appropriate measure for distributed sources is the average geometric efficiency within the sensitive volume for ACD. Midway between the two detectors, this is given by
The term in brackets in Equation 18-7 is the geometric efficiency for a single detector for a point source located at the midpoint of the centerline between the detectors. (As discussed in Chapter 11, Section A.2, this expression is valid when the detector dimensions are “small” in comparison with the source-to-detector distance.) The factor of 2 accounts for the fact that two detectors are used and that if one photon is emitted in a proper direction toward one detector, the other photon is virtually assured of being emitted in the proper direction toward the other. The factor of 1/3 is the average geometric efficiency across the sensitive volume at midplane, that is, the average height of a pyramid.
Actual PET systems typically employ many small detector elements arranged in circular, hexagonal, or octagonal arrays. Each detector element is operated in coincidence with many detectors on the opposing side of the ring, as shown in Figure 18-12. This multicoincidence operation has useful and important consequences for both the magnitude and uniformity of geometric efficiency.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


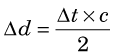
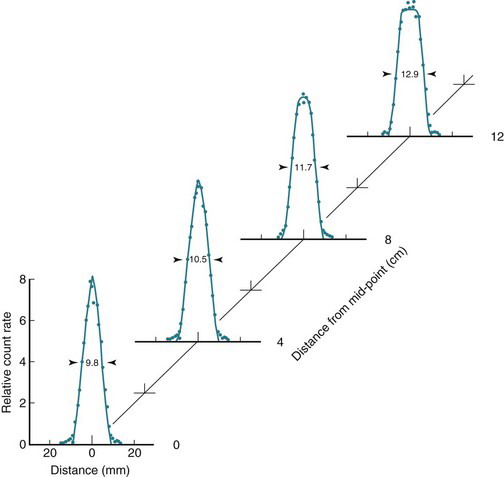
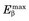 = 0.635 MeV) and 15O (
= 0.635 MeV) and 15O (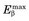 = 1.72 MeV). The profile of the distribution is broader for 15O because of its higher average positron energy, which leads to a longer positron range prior to annihilation.
= 1.72 MeV). The profile of the distribution is broader for 15O because of its higher average positron energy, which leads to a longer positron range prior to annihilation.