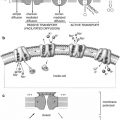
Oncologic
1. Lymphomas and leukemias
2. Polycythemia rubra vera
3. Solid tumors (thyroid carcinoma, neuroblastoma, ovarian, prostate, breast, osteogenic sarcoma, others)
4. Treatment of metastasis-induced bone pain
Non-oncologic
1. Benign thyroid disease particularly hyperthyroidism
2. Radionuclide synovectomy
3. Bone marrow ablation
4. Intravascular radionuclide therapy for prevention of restenosis
It is not the objective of this chapter to discuss different protocols and experiences in the treatment of various conditions using radioisotopes. Rather, the objective is to explore some of the pathological features of the disease processes being treated, the underlying theory behind the action of the radioisotopes that induce therapeutic effects.
Generally, treatment options for cancer may be local (surgery or external beam radiation) or systemic. The role of nuclear medicine focuses on a targeted systemic approach (Fig. 20.1), whether dealing with a primary tumor or with its metastatic foci.
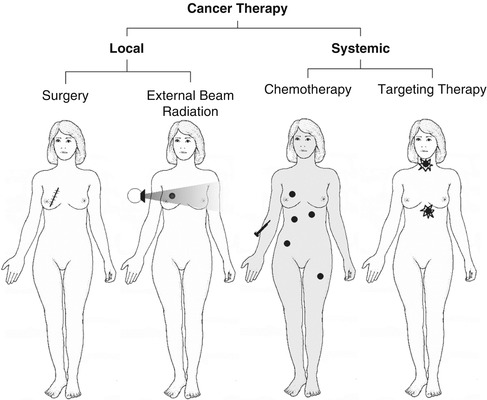

Fig. 20.1
The major types of cancer therapy. Nuclear medicine uses principally the targeting method in treating cancer and cancer metastases (Modified from Prvulovich et al. [1])
20.2 Treatment of Hyperthyroidism
For more than 60 years, iodine-131 has been used to treat most cases of Graves’ disease and hyperfunctioning nodules. It has become the modality of choice in treating Graves’ disease, with the result that surgeons are becoming less and less experienced in thyroidectomy since the number of operations has decreased significantly. In a recent Canadian survey study, endocrinologist were found to be the most common to prescribe I-131 for malignant, while nuclear medicine physicians were the most in prescribing it for benign disease [2].
The normal thyroid gland varies in shape between individuals, and the average weight is approximately 20 g. The gland utilizes iodine for the synthesis of thyroid hormones (see Chap. 7). The cells of the gland do not differentiate between stable iodine and radioactive iodine. Accordingly, if radioactive iodine is administered, it is trapped and then organified by thyroid follicular cells exactly like nonradioactive iodine.
20.2.1 Pathophysiology
After oral administration, I-131 iodide is absorbed rapidly from the upper gastrointestinal tract, 90 % within 60 min. After entering the blood stream, the iodide is distributed in the extrathyroid compartment similar to the stable iodide and leaves this compartment to be taken up by the thyroid and by renal excretion. Approximately 20 % of the administered activity is taken up normally by the thyroid gland. A small amount of I-131 is also found in the salivary glands, gastric mucosa, choroid plexus, breast milk, and placenta. Up to 75 % is excreted by the kidney and 10 % by fecal excretion. Approximately 40 % of the administered activity has an effective half-life of 0.43 days while 60 % has an effective half-life of 7.6 days.
Graves’ disease is the most common form of hyperthyroidism, comprising approximately 56 % of all cases. It is also the major immunologically mediated form. It occurs most commonly in young women and is characterized by symptoms of hyperthyroidism with or without ophthalmopathy and dermopathy. Rarely, lymphadenopathy and splenomegaly may be present. The thyroid gland is usually diffusely enlarged but sometimes normal in size. The condition is an autoimmune process with autoantibodies directed against the TSH receptors on thyroid follicular cells which may be stimulatory and/or destructive [3]. Thyroid stimulatory antibodies include long-acting thyroid stimulator (LATS). This antibody is detected in most patients with Graves’ disease and behaves like TSH, stimulating the production of thyroid hormones and consequently trapping and organifying radioiodine. The other stimulatory antibody is the LATS protector, the antibody that prevents degradation of LATS; accordingly, it helps to stimulate thyroid cells indirectly. The disease is associated with other autoimmune disorders such as pernicious anemia and myasthenia gravis.
Graves’ disease is also known to be associated in Caucasians with HLA B8, DR2, and DR3 and with an inability to secrete certain glycoproteins coded for on chromosomes 6 and 19. A 50 % concordance rate is seen among monozygous twins while 5 % concordance is noted in dizygous twins. These facts suggest a genetic susceptibility for the disease. The observation that Yersinia enterocolitica and Escherichia coli and other gram-negative organisms contain TSH binding sites raised the possibility that the initiating event in the pathogenesis of the disease may be infectious in genetically susceptible individuals.
Histologically, there is hyperplasia of the thyroid epithelium, sometimes with papillary unfolding. Lymphocytic infiltration is present, usually less than in other forms of autoimmune diseases as postpartum thyroiditis. Little colloid storage is also seen. With time, the untreated gland will show progressive fibrosis and the end stage will lead to hypothyroidism, which may be considered part of the natural history of the disease [4, 5].
Thyroid scintigraphy shows uniform uptake throughout the gland or, less commonly, varying degrees of nonuniform uptake. This nonuniformity is related predominantly to different stages of involution of the disease with variable amounts of fibrosis based on the duration of the disease or the presence of nodules (Fig. 20.2). The presence of a TSH-dependent functioning nodule in a diffusely toxic gland has been referred to as Marine–Lenhart’s syndrome (Fig. 20.2). Since the function of such nodule is much less than the surrounding hyperfuctioning tissue, it appears scintigraphically cold.
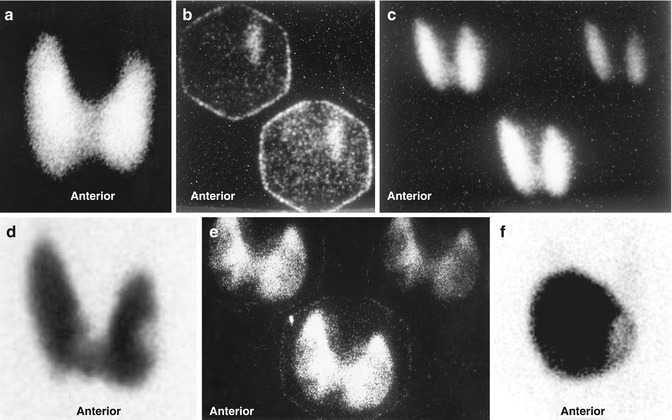

Fig. 20.2
Examples of thyroid scans of patients with hyperthyroidism illustrating patterns that affect the treatment strategy using iodine-131. (a) Illustrates pattern of uniform uptake in a patient with Graves’ disease. Note that scans of patients during recovery phase of thyroiditis may simulate Graves’ disease scintigraphically and show high uptake. Example (b) is of a patient with subacute thyroiditis. Scan shows decreased and nonuniform uptake with a 24-h uptake of 1 %. Follow-up scan (c) shows uniform uptake throughout the gland with an uptake of 38 %. This may be mistaken for Graves’ disease if the patient is referred first during this phase. Example (d) shows diffusely toxic gland with significant nonuniformity and multiple cold nodules. Example (e) shows a scan of a patient with Graves’ disease and a colloid nodule illustrating another pattern of “Marine–Lenhart” syndrome which is more resistant to iodine-131 therapy. Compare this pattern to that of multiple toxic nodules (Fig. 7.2). This pattern also needs to increase activity per gram of tissue for successful treatment. Example (f) is for autonomous single toxic adenoma which is treated by relatively high activity
Ophthalmopathy occurs in approximately 50 % of patients with Graves’ disease [6]. Infiltration of extraocular muscles by an inflammatory reaction consisting predominantly of lymphocytes is the main pathological feature of ophthalmopathy. These lymphocytes are believed to be sensitized to antigens common to the orbital muscles and thyroid gland. Similar inflammatory infiltrates may also be present in the dermis, causing the dermopathy or pretibial myxedema which may be present in up to 10 % of patients with unclear etiology.
Single thyroid nodules can, via an autonomous function, secrete sufficient thyroid hormone to cause hyperthyroidism. These nodules are usually greater than 3 cm in diameter in order to be capable of producing this level of function [7]. Hyperfunction may also arise in a gland containing multiple nodules [8]. In this case, the secretion of thyroid hormones can be either from hyperfunctioning nodules that are assumed to be autonomous or from the internodule parenchyma, which may be an expression of Graves’ disease in an otherwise nodular goiter. The nodules in the latter situation may be cold or a mixture of cold and hot, hypertrophic nodules. The term Plummer’s disease, or toxic nodular goiter, has been used to designate hyperthyroidism in glands with both single and multiple toxic nodules. The term nodular toxic goiter may be reserved for a toxic gland that contains nodules that are not hyperactive. The presence of cancer in toxic nodular goiter is extremely rare and varies from 0.1 to 0.9 %. The toxic nodular goiter may have a cold nodule representing a TSH-dependent adenoma. Scintigraphic imaging cannot exclude malignancy in the cold nodule that is not TSH dependent.
The therapeutic effects of I-131 sodium iodide are due to the emission of ionizing radiation from the decaying radionuclide. In benign conditions such as Graves’ disease, division of some metabolically active cells is prevented by the effect of this ionizing radiation. Cell death is another mechanism activated when the cells are exposed to high levels of radiation, particularly when high doses are given to patients with toxic adenoma, where the suppressed normal thyroid tissue is essentially spared with delivery of a very high concentration to the cells of the toxic nodule. Cell death is followed by replacement with connective tissue, which may lead to hypothyroidism, depending on the number of cells destroyed and replaced by fibrous nonfunctioning tissue. Since 90 % of the radiation effects of I-131 are due to beta radiation, which has a short range in tissue of 0.5 mm, the extrathyroid radiation and consequently the side effects are minimal. It has been estimated that 15 % of patients treated with I-131 may show worsening of ophthalmopathy [9, 10]. Since posttreatment hypothyroidism has been associated with exacerbation of ophthalmopathy, lower-dose radioactive iodine or starting replacement hormones early (2 weeks) after therapy along with the use of prednisone 40–80 mg per day tapered over 3 months may prevent severe eye disease in up to two thirds of patients [11, 12]. It is interesting that cigarette smoking has been also implicated as a risk factor for progression of Graves’ ophthalmopathy [10].
20.2.2 Factors Affecting the Dose of I-131 Used for Therapy of Hypothyroidism
Several factors affect the therapeutic dose to be administered to patients suffering from hyperthyroidism. These include some parameters related to the patient, such as age, sex, medical history, and duration of treatment with antithyroid medications, and factors related to the gland itself, particularly its size, the level of radioiodine uptake, scintigraphic findings of uniform or nonuniform uptake, and whether nodules are present. Additionally, the dose is dependent on how the therapist defines the goals of therapy. If the control of thyrotoxicosis is the most important consideration, the total dose or the dose per gram of estimated thyroid tissue weight will be higher than when the therapist is trying to avoid or delay hypothyroidism [13]. Using empirical low-dose iodine therapy to avoid hypothyroidism has been shown to result in persisting hyperthyroidism in up to 54 % of patients [14]. Additionally, it has been found that the rate of hypothyroidism is not different among those treated with low-dose and high-dose radioiodine [15, 16].
20.3 Treatment of Differentiated Thyroid Cancer
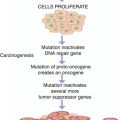
Radioactive iodine is the mainstay of therapy for residual, recurrent, and metastatic thyroid cancer that takes up iodine and cannot be resected. The tissue of normal thyroid and its tumors expresses a variety of oncogenes, growth factors, and growth factor receptors. There is increased expression of some oncogenes, namely, c-myc/c-fos and c-ras, in some epithelial and medullary thyroid carcinomas.
C-myc mRNA and c-fos mRNA are found in high levels in papillary carcinomas compared with the surrounding normal thyroid tissue. Patients with an unfavorable prognosis were twice as likely to overexpress c-myc as patients with good prognosis [17].
Ras oncogenes were found in 80 % of follicular and 20 % of papillary carcinomas. This high prevalence of transforming ras oncogenes in follicular carcinomas may explain its aggressive behavior in comparison to papillary carcinoma and may suggest a role of this oncogene in the metastatic phenotype of this cancer [18]. Recently a tissue-specific oncogene associated with papillary carcinoma has been identified.
Excessive growth factor and increased expression of oncogenes encoding growth factors or growth factor expression, such as the oncogene of c-ras B were identified in papillary carcinoma, adenomas, and anaplastic carcinoma.
Besides the importance of growth factors in the development of thyroid carcinoma, links have also been found to certain risk factors. The most important of these is radiation exposure. Exposure to radiation following the explosion of the atomic bombs in Japan, as well as after head and neck radiation, resulted in a 30-fold increase in the incidence of thyroid cancer [19].
About 90 % or more of thyroid carcinomas are well differentiated, of the papillary, papillofollicular, follicular, and Hürthle-cell types, which take up iodine and accordingly can be successfully treated with I-131. The therapeutic effects on differentiated thyroid cancer, where larger doses of radioactive iodide are administered, are based on destruction of cells of the residual thyroid tissue and the functioning carcinoma cells by the high dose of administered radionuclide. The mortality of patients treated with less than total thyroidectomy and limited I-131 therapy was found to be three to four times higher than that of patients treated with total thyroidectomy and I-131 therapy to ablate known foci of radioiodine uptake [20] (Fig. 20.3). Because of the larger dose of radionuclide and the lower uptake by the tissue in the case of thyroid cancer, more side effects can be seen, particularly transient sialadenitis, than in treatment of hyperthyroidism. This however does not justify using limited therapy such as 30 mCi. A recent study confirmed the high rate of efficiency of the high ablative dose of 100 mCi of I-131 particularly in patients with less than 2 % neck uptake values [21]. This study confirmed also that success rate is dependent on the pre-therapy neck uptake. The success rate was 94 % when pre-ablation uptake was less than 2, 80 % with uptake between 2 and 5, and 60 % when uptake value was more than 5 % [21].
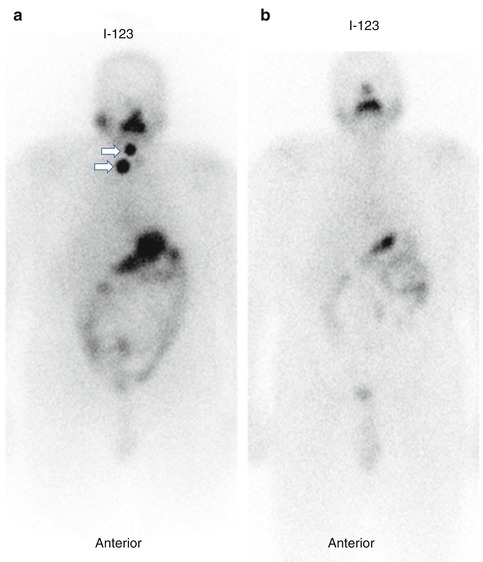

Fig 20.3
123I whole body scan (a) for a patient with papiary thyroid carcinoma treated with total thyroidectomy. The scan shows neck activity (arrows). Foow up scan (b) one year after I-131 ablation shows complete resolution. Follow-up I-123 whole-body scan in a patient with papillary thyroid carcinoma treated with total thyroidectomy and I–131 ablation showing resolution of the neck activity 1 year after I-131 postoperative ablation
Thyroglobulin and calcitonin are the major tumor markers for thyroid cancer of the follicular epithelium and parafollicular C cells, respectively. These markers are unique, in the sense that they are not only specific for tumor tissue but are also specific components of normal thyroid tissue. Thyroglobulin is an iodinated glycoprotein essential for synthesis and storage of thyroid hormones. Since thyroglobulin is produced exclusively by thyroid tissue, only very small amounts can be found in the blood after thyroidectomy and ablative radioiodine therapy. Accordingly, any post-therapeutic elevation of its levels indicates either remnant thyroid tissue, requiring further ablative treatment, or the presence of metastases or local recurrence. Other tumor markers used for many other tumors, such as carcinoembryonic antigen (CEA) and tissue polypeptide antigen (TPA), are not specific for thyroid cancer. TPA, which is a cytokeratin-related nonspecific proliferation marker, has a sensitivity of 40–60 % for thyroid cancer. However, it has a good correlation with tumor progression or therapeutic response, with a high positive predictive value of 90 %. Evaluation of ablative therapy and follow-up of patients post ablation to monitor disease recurrence has further improved and facilitated by the availability of recombinant human thyrotropin as well as the use of F-18 FDG positron emission tomography. The value of recombinant human thyrotropin (rhTSH) rests on providing the opportunity to obtain diagnostic whole-body I-131 scan under adequate TSH elevation as well as representative thyroglobulin levels while the patients receiving their thyroid hormone [22]. FDG-PET is useful in evaluating patients in instances where radioiodine imaging fails to identify known or suspected recurrent or metastatic disease [23]. Additionally, the use of Tl-201 and Tc99m MIBI particularly when FDG-PET is not available is of value for this purpose [24].
20.4 Treatment of Pain Secondary to Skeletal Metastases
Approximately 75 % of patients with advanced cancer have pain, with a high percentage due to skeletal metastases. Bone metastases cause intractable pain, which affects the quality of life for the patient, especially if it is associated with immobility, anorexia, and anxiety, with the consequent long-term use of narcotic analgesics. The mechanism of bone pain may not be clear in many of these patients and could be due to cell-secreted pain modulators such as interleukin-1 beta, interleukin-8, and interferon [25]. Depending on the extent of bone metastases, radiation therapy or radiopharmaceuticals can be used instead of narcotics to alleviate the pain with the objective of improving the quality of life.
Radiotherapy for focal painful metastases with delivery of 2,000–3,000 rads induces pain relief in 60–90 % of cases [26, 27]. Controlling pain of multiple metastases using external beam radiotherapy is difficult. Hemibody irradiation using 800 rads to the lower half of the body and 600 rads to the upper half has resulted in complete response in 30 %, partial response in 50 %, and no response in 20 % of patients. Radiotherapy used for painful skeletal metastases often produces significant side effects such as nausea, vomiting, and diarrhea, as well as bone marrow toxicity in one third of patients. Vomiting and diarrhea can be severe in 10 % of cases and hematological side effects can be life threatening in approximately 9 % of patients [28].
Bone-seeking radiopharmaceuticals emitting beta particles have been used to deliver local radiotherapy to metastases to decrease pain at their sites. Radiopharmaceuticals which are taken up at the sites of bone metastases will cause less toxicity than external radiation therapy. These radiopharmaceuticals control pain while causing only transient bone marrow depression, which is usually mild. The uptake of these radiopharmaceuticals by metastases is severalfold (up to 15–20 times) that of normal bone. These agents are absorbed to hydroxyapatite crystals at the site of active new bone, similar to Tc99m-MDP. They include phosphorus-32, strontium-89, rhenium-186 diphosphonate, and samarium-153 EDTMP. The list of radiopharmaceuticals for bone palliation has been increasing including Re-188, Lu-177, and others [29].
20.4.1 Radiopharmaceuticals
20.4.1.1 Strontium-89 Chloride (Sr-89 Chloride)
Systemic radionuclide therapy with Sr-89 chloride was first used to relieve pain from bone metastases in1937 and regained popularity in the 1980s. It is a pure beta emitter with a relatively long half-life of 50.5 days. It is a chemical analogue of calcium, and accordingly it concentrates avidly in areas of high osteoblastic activity. After intravenous injection, strontium quickly accumulates in the mineral bone matrix where active bone formation takes place. Therefore, there is preferential uptake in and around metastatic tumor deposits which has been confirmed by external measurements using the gamma emitting radionuclide Sr-85 and by autoradiography. It was found that Sr-89 concentration is 2–20 times greater in bone metastases than normal bone [30]. The biological half-life of Sr-89 in bone lesions is about 90 days, compared to about 2 weeks in normal bone which can be explained by the immature nature of reactive bone compared to normal lamellar bone. This selective uptake and prolonged retention at sites of increased bone mineral turnover provide precise targeting of bone lesions. The radionuclide is typically administered as a single 150 MBq (4 mCi) intravenous dose. Overall, pain relief occurs in up to 80 % of patients, of whom 10–40 % became effectively pain free. The mean duration of palliation is 3–4 months [31, 32]. Furthermore, 89Sr-chloride may cause slowing of metastatic progression due to inhibition of expression of cell adhesion molecules (E-selectins) that participate in the metastatic process. The significant transient decrease in serum E-selectin concentration as observed after systemic radionuclide therapy in a study on 25 men with metastatic prostate carcinoma is an indication of such an observation [33] and may provide opportunities for clinical trials.
20.4.1.2 Samarium-153 Ethylenediaminetetramethylene Phosphonate (Sm-153-EDTMP)
Samarium-153 is produced in the nuclear reactor by neutron activation of both natural Sm-2O3 and 98 % enriched Sm-152 targets. It has a relatively short half-life of about 48 h. Coupling of the radionuclide to ethylenediaminetetramethylene phosphonate (EDTMP) leads to the high uptake of the radionuclide by bone. Gamma camera imaging is possible due to the 103 KeV gamma ray emitted during decay of Sm-153. The resulting images are similar to those obtained with Tc99m-MDP or other diphosphonates showing increased uptake at the site of metastases. The calculated lesion to normal-bone ratio was reported to be 4.0 and to soft-tissue ratio to be 6.0 [34].
Administration of 153Sm-EDTMP according to the supplier’s recommendations at 37 MBq (1 mCi)/kg would deliver a bone marrow dose of 3.27–5.90 Gray (Gy) which would induce myelotoxicity as a side effect. Dosimetric calculation by urine collection and whole-body scintigraphy has been used to limit the bone marrow dose to 2 Gy by Cameron and associates [35]. This was achieved by anterior and posterior whole-body images obtained 10 min and 5 h after the intravenous injection of 740 MBq (20 mCi) of 153Sm-EDTMP with determination of bone activity by imaging and by counting urine collected for 5 h. The total administered activity of 153Sm-EDTMP predicted on a 2 Gy bone marrow dose was found to be 35–63 % of the standard recommended dose of 37 MBq/kg. The authors reported pain relief in eight of the ten patients treated using this dosimetric method [35].
20.4.1.3 Rhenium-186 Ethylene Hydroxy Diphosphonate (Re-186-EHDP)
Similar to Sm-153, Re-186 has been coupled to a bone-seeking phosphonate, ethylene hydroxy diphosphonate (EHDP). This radionuclide emits beta particles with a maximum energy of 1.07 MeV and gamma photons with an energy of 137 KeV which allows bone scanning. Re-186-EHDP undergoes renal excretion within 6 h after intravenous injection, as is the case with the common bone-scanning agents. At 4 days, 14 % of the radioactivity remains in bone [36].
Several studies have shown encouraging clinical results of palliative therapy using 186Re-HEDP with an overall response rate of approximately 70 % for painful osseous metastasis from prostate and breast cancer. Myelosuppression has been limited and reversible, which makes repetitive treatment safe [37, 38]. In a study of 31 patients with various cancers (10 prostate, 10 breast, 4 rectum, 5 lung, 2 nasopharynx) and bone metastases treated with a fixed dose of 1,295 MBq (35 mCi) of Re-186 HEDP. When necessary, the same dose was repeated two to three times after an interval of 10–12 weeks. The mean response rate was 87.5 % in patients with breast and prostate cancer, 75 % in patients with rectal cancer, and 20 % in patients with lung cancer. The overall response rate was 67.5 % and the palliation period varied between 6 and 10 weeks. The maximal palliation effect was observed between the 3rd and 7th weeks [38].
20.4.1.4 Tin-117m- Diethylenetriaminepentaacetic Acid (Sn-117m-DTPA)
Tin-117m is a reactor produced radionuclide, with a half-life of 13.6 days. Contrary to the other radionuclides mentioned above, this radionuclide emits internal conversion electrons. Tin-117m is linked to diethylenetriaminepentaacetic acid (DTPA). More than 50 % of the administered activity is absorbed by bone in patients with metastatic carcinoma with a bone to red marrow ratio of up to 9:1. Its 159 KeV photon energy allows correlative imaging with a similar uptake pattern as Tc99m-MDP [39].
In a preliminary study in 10 patients by Atkins et al. [40], none of the patients who received Sn-117m-DTPA for palliation developed marrow toxicity. Another recent study on 47 patients treated with Sn-117-DTPA showed that the experimental mean absorbed dose to the femoral marrow was 0.043 cGy/KBq. In comparison to P-32-orthophosphate, Sn-117m-DTPA yielded up to an eightfold therapeutic advantage over the energetic beta emitter P-32. Accordingly, it was suggested that internal conversion electron emitter Sn-117m offers a large dosimetric advantage over the energetic beta-particle emitters allowing higher administered activity for alleviating bone pain, while minimizing marrow toxicity [41].
20.4.1.5 Phosphorus-32 Orthophosphate
This radionuclide is used uncommonly for the treatment of bone metastases. Dosimetric studies have demonstrated a relatively high dose to the bone marrow from the highly energetic beta particles of this radionuclide causing myelosuppression with pancytopenia. Increased incidence of acute leukemia has been reported although this was reported following P-32-therapy in patients with polycythemia vera.
20.4.1.6 Rhenium-188 Dimercaptosuccinic Acid Complex [Re-188(V)DMSA]
Re-188(V)DMSA, a potential therapeutic analogue of the tumor imaging agent Tc99m(V)DMSA, is selectively taken up in bone metastases. In a study by Blower PJ et al. [42] on ten patients with metastatic prostate cancer studied by Tc99m(V)DMSA and 188Re(V)DMSA to compare their biodistribution, only minor differences between both radiopharmaceuticals were found. Accordingly, Tc99m(V)DMSA scans are predictive of 188Re(V)DMSA biodistribution and could be used to estimate tumor and renal dosimetry and assess suitability of patients for Re-186(V)DMSA treatment [42]. This advantage makes this tracer a candidate for more trials as a potentially successful agent for bone metastases palliation.
20.4.2 Mechanism of Action
Metastatic bone pain is believed to be due to mechanical factors due to local bony destruction and to humoral factors resulting from secretion of certain mediators by tumor and peri-tumoral cells (Table 20.2). Although the mechanism of action of these radiopharmaceuticals in relieving bone pain is not completely known, the therapeutic effect is thought to be achieved by delivering sufficient energy from the sites of reactive bone directly to the cells of metastases and/or to peri-tumor cytokine-secreting cells that may be responsible for the patient’s pain. Pain relief by radiation was found to be independent of the radiosensitivity of the tumor and therefore the mechanism of action does not involve actual killing of the tumor cell. It is more likely that radiation interrupts processes that are maintained by humoral pain mediators in the microenvironment of the tumor [43]. This view is also supported by absence of a dose-response relationship [44].
Table 20.2
Types of cellular damage in relation to approximate radiation dose
Dose [Grays (rads)] | Type of damage | Comments |
|---|---|---|
0.01–0.05 (1–5) | Mutation (chromosomal aberration, gene damage) | Irreversible chromosome breaks, may repair |
1 (100) | Mitotic delay, impaired cell function | Reversible |
3 (300) | Permanent mitotic inhibition, impaired cell function, activation and deactivation of cellular genes and oncogenes | Certain functions may repair; one or more divisions may occur |
>4–10 (>400–1,000) | Interphase death | No division |
500 (50,000) | Instant death | No division |
Proteins coagulate |
20.4.3 Choice of Radiopharmaceutical
It has been demonstrated that myelosuppression is less severe using radionuclides with relatively shorter half-lives favoring the use of Sm-153, Re-186, Sn-117, and Sr-89. Other physical properties including radiolabeled conjugate biological uptake and clearance, product-specific activity, range and type of emissions, and resultant effects on tumor and normal tissue cellular survival should be all considered along with the clinical outcome to choose a radiopharmaceutical. The response rate of different radiopharmaceuticals currently in use appears not to differ significantly [45]. The side effects which are mainly hematological vary among the agents used, being more pronounced with P-32 than with the newer agents. Tin-117m DTPA differs from the other radiopharmaceuticals in that its emission is internal conversion electrons rather than beta particles. Since internal conversion electrons have low energy and shorter path in tissue, they may result in less marrow toxicity.
20.4.4 Clinical Use
Radiopharmaceutical therapy is indicated for the treatment of patients with painful widespread bone metastases. However, the patient with pain secondary to either spinal cord or peripheral nerve invasion by adjacent metastases will not benefit from such treatment. The contraindication in pregnancy is absolute, and relative contraindications include preexisting severe myelosuppression, urinary incontinence, severe insufficiency, and spinal cord compression or pathological fracture. A pre-therapy bone scan, neurological examination, and blood counts should be available before the patient is treated. Follow-up blood counts should be performed at least biweekly to evaluate myelotoxicity. The response to these radiopharmaceuticals is more or less similar, with an average success rate of 70–80 % [46–52].
The difference in half-life of the radiopharmaceuticals and the extent of bone metastases have consequences for both the onset and the duration of pain relief. Relief rates using the newer agents are not significantly different and are comparable with those of external beam radiotherapy, but side effects are minimal and compare favorably with those of the older agent P-32.
Using radionuclide along with chemotherapy for palliation is being investigated and may proof useful. Palmedo et al. reported a case of a patient with disseminated bone metastases due to breast cancer and multifocal pain. Because of persisting pain after a first cycle of chemotherapy, 1,295 MBq Re-186 HEDP was administered and pain relief was significant. Subsequently, the patient received combined chemotherapy along with Re-186 HEDP therapy and remained pain free. Follow-up Tc99m-MDP bone scan showed significant regression of osseous metastases. The authors speculated that the combination of Re-186 HEDP and chemotherapy resulted in significantly increased palliation of metastatic bone disease [53].
The side effects, which are mainly hematological, vary among the agents used, being more pronounced with P-32 than with the newer agents. Some agents have the advantage of emitting gamma energy suitable for scintigraphy such as samarium-153 EDTMD (ethylenediaminetetramethylene phosphonate). Tin-117m DTPA differs from the other radiopharmaceuticals in that it emits conversion electrons rather than beta particles. These conversion electrons have low energy and a shorter path in tissue and may then result in less marrow toxicity [50, 54].
20.5 Treatment of Neuroendocrine Tumors
Neuroendocrine tumors constitute a heterogeneous group of neoplasms originating from endocrine cells that secrete biogenic amines and polypeptide hormones. Recently, the incidence of these tumors has gradually increased worldwide. The clinical behavior of neuroendocrine tumors is significantly variable; they may be hormonally active or nonfunctioning, ranging from very slow-growing tumors to highly aggressive and very malignant tumors. Surgery is currently the only available curative treatment for these tumors, but for patients who have inoperable primary, recurrent or metastatic disease, few therapeutic options are available. The goals of radionuclide therapy for neuroendocrine tumors are to control symptoms and pain, improve the quality of life, reduce medical requirements, and stabilize the disease. Additionally, in limited disease it is used to reduce tumor volume, reduce hormone secretion, and help complete remission.
Several neuroendocrine tumors are candidates for radionuclide therapy. I-131 has been used to treat neuroblastoma, pheochromocytoma, and paraganglioma. More recently octreotide and other analogues labeled with In-111, Y-90, and Lu-177 are being used [55–57] (see later).
I-131 metaiodobenzylguanidine (MIBG) is being used for the treatment of pheochromocytoma, malignant paraganglioma, neuroblastoma, medullary thyroid carcinoma, and symptomatic carcinoid tumors. The radiopharmaceutical resembles guanethidine and is concentrated by normal and abnormal sympathetic adrenergic tissue.
When I-131 MIBG is administered intravenously, it is transported by blood to be taken up by normal adrenergic tissue such as the adrenal medulla and sympathetic nervous system and by tumors of neuroectoderm-derived tissue. The uptake by these tumors is secondary to active uptake-1 mechanism and passive diffusion through the cell membrane, followed by active intracellular transport to the neurosecretory granules in the cytoplasm, where it is retained.
In normal adrenergic tissue such as the adrenal medulla, heart, and salivary glands, as well as in pheochromocytoma, 90 % of MIBG is stored in the neurosecretory granules, while in neuroblastoma it was found that up to 60 % is stored within the extragranular cells. The major part of the radiopharmaceutical is excreted unchanged in urine. Other than in the adrenergic tissues, uptake is normally noted in the liver, spleen, urinary bladder, bowel, lungs, nose, near the trapezium muscle in children, and in the uterus in some women [58, 59]. The radiation effect is due to emission of beta particles from the decaying I-131 with a mechanism similar to that in treating thyroid disorders. A long list of medications is known to block the uptake and/or retention of MIBG by the target tissues, while some reports have suggested that others such as calcium channel blockers may increase its uptake. The mechanism of interference of these drugs varies. Beta-blockers, for example, interfere with the uptake by inhibiting the uptake mechanism-1 and by depleting the neurosecretory granules, while reserpine exerts this action by depleting the granules and inhibiting the intracellular transport. More recently peptide therapy has been increasingly used to treat these tumors (shown later in the chapter).
20.5.1 Neuroblastoma
Therapeutic amounts of I-131 MIBG can be delivered to neuroblastoma with acceptable bone marrow toxicity [60–62]. Among patients with stages 3 and 4 neuroblastoma who had failed treatment with chemotherapy, I-131 MIBG induced partial remission in many children and complete remission in a small number of patients. The agent has also been used for early therapy at the time of diagnosis, with a success rate comparable to that of chemotherapy with fewer side effects [61]. Since some neuroblastomas express somatostatin receptors, peptide receptor radionuclide therapy particularly with 177Lu-DOTA-TATE is also beneficial.
20.5.2 Pheochromocytoma
Malignant pheochromocytoma and its metastases are known to be resistant to chemotherapy and external beam radiation therapy. I-131 MIBG has a limited role in the treatment of malignant pheochromocytoma, functioning paraganglioma, and medullary carcinoma of the thyroid. Palliative effects have been achieved in patients with pheochromocytoma [63]. Several reports from the USA and Europe have collectively shown a response of 62.5 % among patients with pheochromocytoma [45]. Soft-tissue metastases responded better than skeletal metastases.
20.5.3 Carcinoid Tumor
Carcinoid liver metastases are common and rarely can be resected. Treatment for symptomatic patients with unresectable disease includes chemotherapy, interferon alpha, and the somatostatin analogue, octreotide. The response to these medical therapies is usually poor. Hepatic artery ligation and embolization are alternatives and have a better response rate. Preliminary experience also suggests that external beam radiotherapy can be useful. I-131 MIBG and radiolabeled octreotide have recently been tried. I-131 MIBG is highly concentrated by more than 60 % of carcinoid metastases. Carcinoid tumor cells stain positive for chromogranin A [64]. I-131 MIBG targets the metabolically active lesions, reduces the hormonal secretion, and improves symptoms [1, 65]. Data indicate a partial response in 20 % of patients and a palliative effect in more than 50 % of those with end-stage disease. I-131 MIBG causes temporary myelosuppression, which makes its use favorable compared with chemotherapy. It is also preferred to interferon alpha and octreotide, which require frequent subcutaneous injections.
Pathologically, I-131 MIBG produces gross cystic changes in liver metastases which probably are due to ischemic necrosis. Surgical deroofing and aspiration of cysts can lead to regeneration of normal liver tissue [1].
20.6 Radioimmunotherapy
Monoclonal antibodies are now contributing increasingly to cancer treatment, following early disappointments. I-131 anti-CD-20 and I-131 anti-CD-22 are good examples which are used for non-Hodgkin’s lymphomas. These antibodies can be used alone to kill tumor cells or conjugated with drugs, cytotoxic agents, and radionuclides to improve their effects.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree