Benign Tumors and Tumor-Like Lesions II: Lesions of Cartilaginous Origin
Benign Chondroblastic Lesions
Diagnosis of a bone lesion as originating from cartilage is usually a simple task for the radiologist. The lesion’s radiolucent matrix, scalloped margins, and annular, comma-shaped, or punctate calcifications usually suffice to establish its chondrogenic nature. However, whether a cartilage tumor is benign or malignant is sometimes extremely difficult for the radiologist to determine.
Enchondroma (Chondroma)
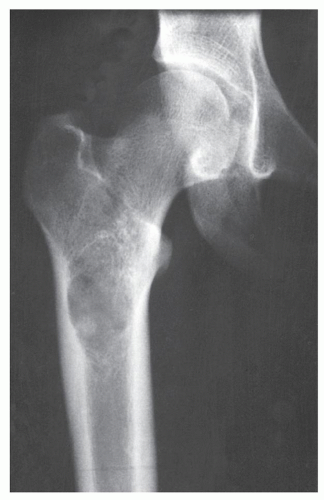
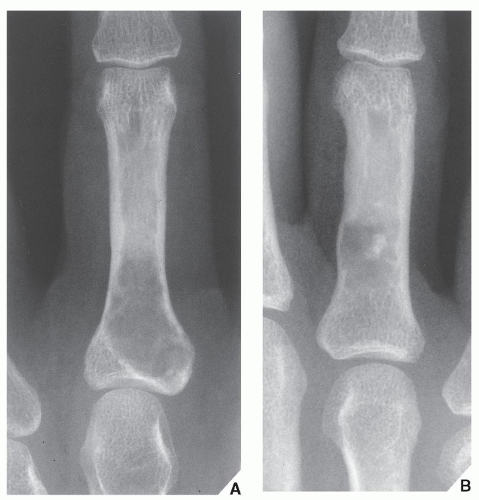
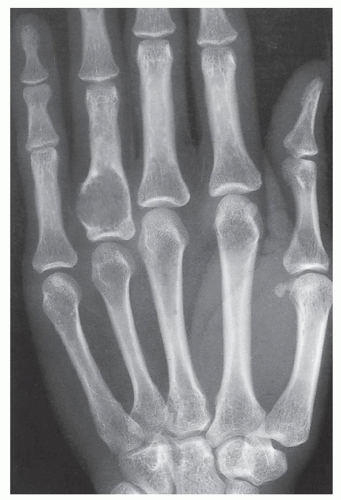
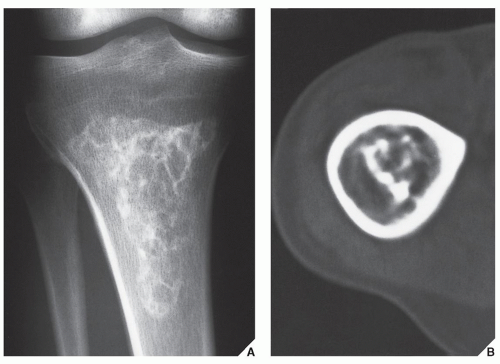
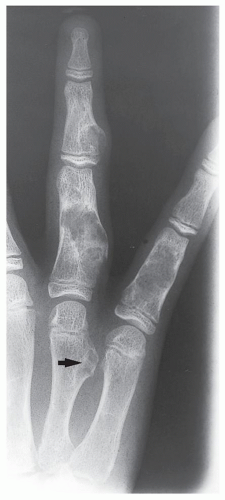
Enchondroma is the second most common benign tumor of bone, constituting approximately 10% of all benign bone tumors and representing the most common tumor of the short tubular bones of the hand. When the lesion is located centrally in the bone, it is termed an enchondroma (Fig. 18.1); if it is extracortical (periosteal) in location, it is called a chondroma (periosteal or juxtacortical) (see Figs. 18.10 and 18.11). Regardless of location, this benign lesion is characterized by the formation of mature hyaline cartilage. It has been widely postulated that enchondroma is formed as a consequence of displacement of embryonic rests of cartilage from the growth plate into the metaphysis. This contention, however, was recently challenged by some investigators whose work failed to confirm this theory. Furthermore, the study by Amary and colleagues identified somatic mutations in isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) in many central low-grade cartilaginous tumors, thus supporting the neoplastic origin of enchondromas. Moreover, most chondromas contain clonal chromosomal abnormalities involving chromosomes or chromosomal regions 4q, 5, 7, 11, 14q, 16q22-q24, 20, and particularly rearrangement of chromosome 6 and 12q12-q15. Although occurring throughout life, enchondromas are usually seen in patients in their second through fourth decades. There is no sex predilection. The short tubular bones of the hand (phalanges and metacarpals) are the most common sites of occurrence (Fig. 18.2), although the lesions are also encountered in the long tubular bones (Fig. 18.3). Sporadic cases have been reported in the rib, clavicle, cuboid, and carpal bones. They are often asymptomatic; a pathologic fracture through the tumor (Figs. 18.4 and 18.5) often calls attention to the lesion.
Enchondroma protuberans is a rare variant. It is a lesion that arises in the intramedullary cavity of a long bone and forms a prominent exophytic mass on the cortical surface. This lesion must be distinguished from an osteochondroma or central chondrosarcoma that penetrates the cortex and forms a juxtacortical mass.
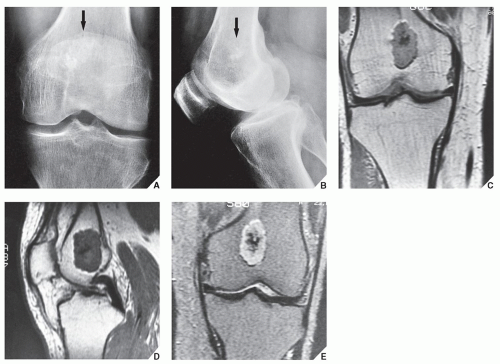
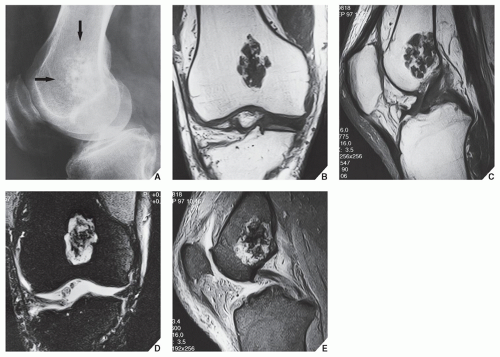
In most instances, radiography suffices to demonstrate the lesion. In the short bones, the lesion is often entirely radiolucent (Fig. 18.6), whereas in the long bones, it may display visible calcifications. If the calcifications are extensive, enchondromas are called calcifying (Fig. 18.7). The lesions can also be recognized by shallow scalloping of the inner (endosteal) cortical margins because the cartilage in general grows in a lobular pattern (see Fig. 18.1).
Computed tomography (CT) and magnetic resonance imaging (MRI) may further delineate the tumor and more precisely localize it in the bone. On spin echo T1-weighted MR images, enchondromas demonstrate intermediate to low signal intensity, whereas on T2-weighted images, they exhibit high signal intensity. The calcifications within the tumor will image as low-signal intensity structures (Figs. 18.8 and 18.9). It must be stressed, however, that most of the time neither CT nor MRI is suitable for establishing the precise nature of a cartilaginous lesion, nor can CT or MRI distinguish benign from malignant lesions. Despite the use of various criteria, the application of MRI to the tissue diagnosis of cartilaginous lesions has not brought satisfactory results, although preliminary results of recent trials with fast contrast-enhanced MR imaging showed that this technique might assist in differentiation between the benign and malignant cartilaginous tumors.
Skeletal scintigraphy usually reveals mild to moderate increased uptake of the tracer in uncomplicated enchondromas, whereas the presence of a pathologic fracture or malignant transformation is revealed by marked scintigraphic activity.
Intracortical chondroma is a very rare variant of conventional enchondroma. The lesion is located in cortical bone and is surrounded by sclerosis of the medullary bone and periosteal reaction. Some of these lesions may actually represent periosteal chondroma with an atypical radiographic appearance, as reported by Abdelwahab and associates. Intracortical chondroma can occasionally simulate an osteoid osteoma.
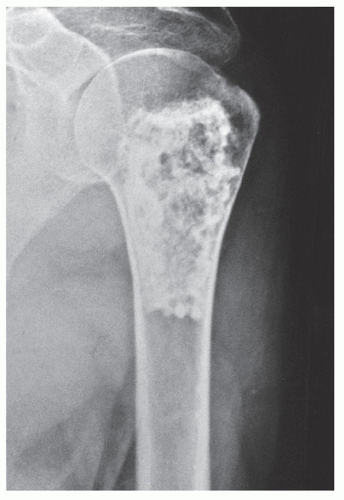
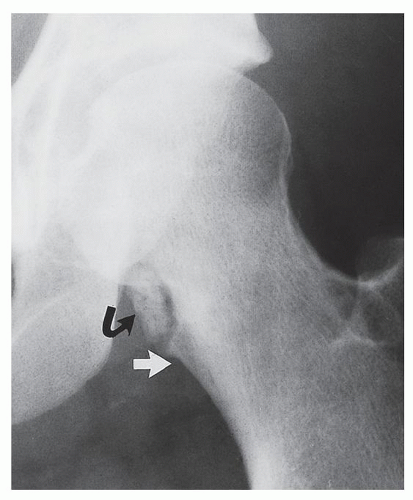
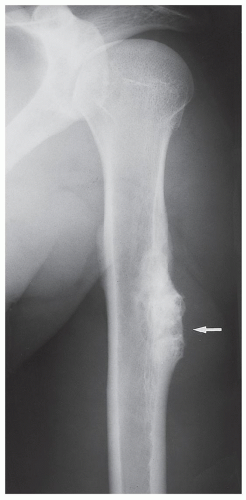
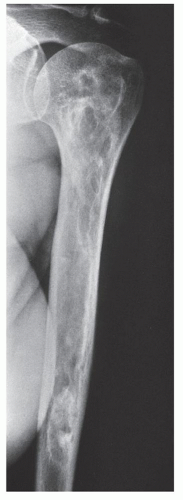
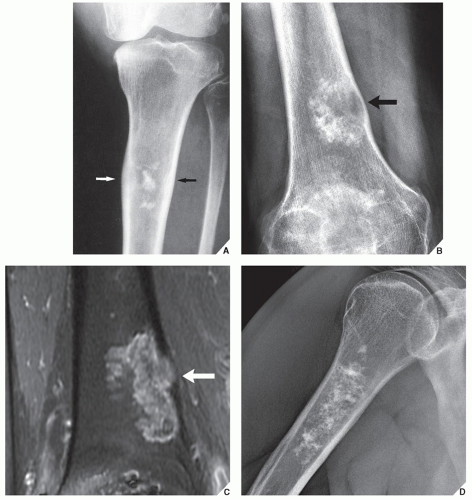
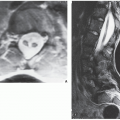
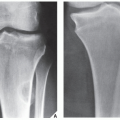
Periosteal chondroma is a slow-growing, benign cartilaginous lesion that arises on the surface of a bone in or beneath the periosteum. It occurs in children as well as adults, with no sex predilection. There is usually a history of pain and tenderness, often accompanied by swelling at the site of the lesion, which is most commonly located in the proximal humerus. As the tumor enlarges, it is seen radiographically eroding the cortex in a saucer-like fashion, producing a solid buttress of periosteal new bone (Fig. 18.10). The lesion has a sharp sclerotic inner margin demarcating it from the buttress of periosteal new bone. Scattered calcifications are often seen within the lesion (Fig. 18.11).
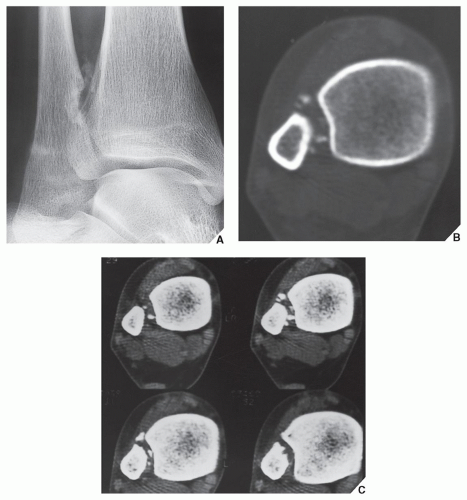
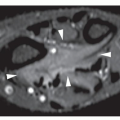
CT may show to better advantage the scalloped cortex and matrix calcification (Fig. 18.12). It also may demonstrate the separation of a lesion
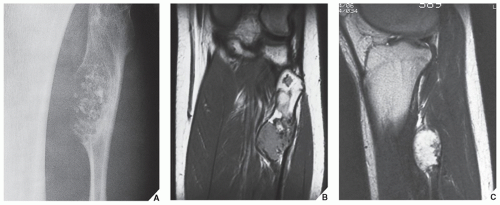
from the medullary cavity, an important feature in differentiation from osteochondroma. MRI findings correspond to radiographic findings, depicting the cartilaginous soft-tissue component. If periosteal chondroma affects the medullary canal, MRI may be useful in depicting the extent of involvement (Fig. 18.13). Fat suppression or enhanced gradient-echo sequences may improve tumor-marrow contrast. The potential pitfall of MRI is marrow edema mimicking tumor invasion or vice versa. Unlike enchondroma and osteochondroma, periosteal chondroma may continue to grow after skeletal maturation. Some lesions may attain a large size (up to 6 cm) and may resemble osteochondromas (Figs. 18.14 and 18.15). Some lesions may mimic an aneurysmal bone cyst. Very rarely, the lesion may encase itself intracortically, thus mimicking other intracortical
lesions (such as intracortical angioma, intracortical fibrous dysplasia, or intracortical bone abscess).
from the medullary cavity, an important feature in differentiation from osteochondroma. MRI findings correspond to radiographic findings, depicting the cartilaginous soft-tissue component. If periosteal chondroma affects the medullary canal, MRI may be useful in depicting the extent of involvement (Fig. 18.13). Fat suppression or enhanced gradient-echo sequences may improve tumor-marrow contrast. The potential pitfall of MRI is marrow edema mimicking tumor invasion or vice versa. Unlike enchondroma and osteochondroma, periosteal chondroma may continue to grow after skeletal maturation. Some lesions may attain a large size (up to 6 cm) and may resemble osteochondromas (Figs. 18.14 and 18.15). Some lesions may mimic an aneurysmal bone cyst. Very rarely, the lesion may encase itself intracortically, thus mimicking other intracortical
lesions (such as intracortical angioma, intracortical fibrous dysplasia, or intracortical bone abscess).
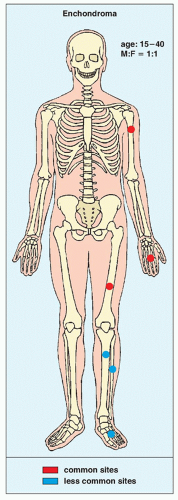 FIGURE 18.3 Skeletal sites of predilection, peak age range, and male-to-female ratio in enchondroma. |
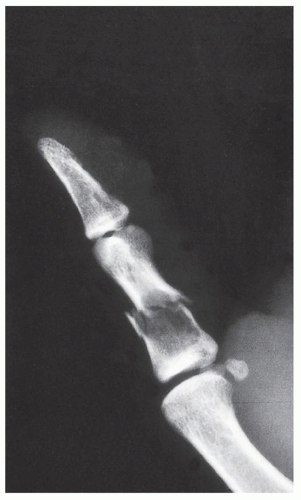 FIGURE 18.4 Enchondroma. Radiograph of a 31-year-old man who had injured his left thumb reveals a pathologic fracture through an otherwise asymptomatic lesion. |
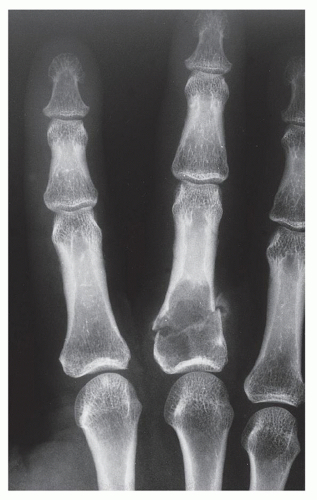 FIGURE 18.5 Enchondroma. Pathologic fracture through a large enchondroma is present in the proximal phalanx of the middle finger. |
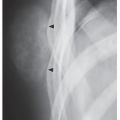
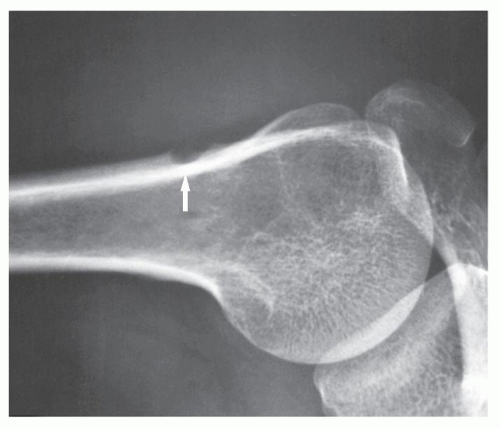 FIGURE 18.10 Periosteal chondroma. A radiolucent lesion (arrow) is eroding the external surface of the cortex of the proximal humerus of a 24-year-old man. |
Histologically, enchondroma consists of lobules of hyaline cartilage of varying cellularity and is recognized by the features of its intracellular matrix, which has a uniformly translucent appearance and contains relatively little collagen. The tissue is sparsely cellular, and the cells contain small and darkly staining nuclei. The tumor cells are located in rounded spaces known as lacunae. On histologic examination of periosteal chondroma, the findings are identical to those of enchondroma, although the lesion sometimes exhibits higher cellularity, occasionally with atypical cells.
Differential Diagnosis
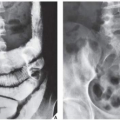
The main differential diagnosis of enchondroma, particularly in lesions of the long bones, is a medullary bone infarct (Fig. 18.16). At times, the two lesions may be difficult to distinguish from one another, particularly if the enchondroma is small, because both lesions present with similar calcifications. The radiographic features helpful in the differential diagnosis are the lobulation of the inner cortical margins in enchondroma, the annular, punctate, and comma-shaped calcifications in the matrix, and the lack of sclerotic rim that is usually seen in bone infarcts (Fig. 18.17).
The most difficult task for the radiologist is to distinguish a large solitary enchondroma from a slowly growing low-grade chondrosarcoma. One of the most significant findings pointing to a chondrosarcoma in the early stage of development is localized thickening of the cortex and deep endosteal scalloping (Fig. 18.18). The size of the lesion should also be taken into consideration. Lesions longer than 4 cm (or, according to some investigators, longer than 7 cm) are suggestive of malignancy. In more advanced tumors, destruction of the cortex and the presence of a soft-tissue mass are the hallmarks of malignancy.
Complications
The single most important complication of enchondroma, aside from pathologic fracture (see Fig. 18.4), is its malignant transformation to chondrosarcoma. With solitary enchondromas, this occurs almost exclusively in a long or flat bone and almost never in a short tubular bone. The radiographic signs of the transformation are thickening of the cortex, destruction of the cortex, and a soft-tissue mass. The development of pain in the absence of fracture at the site of the lesion is an important clinical sign.
Treatment
Curettage of the lesion with the application of bone graft is the most common course of treatment.
Enchondromatosis, Ollier Disease, and Maffucci Syndrome
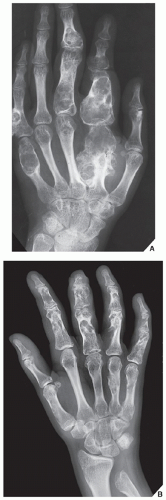
Enchondromatosis is a condition marked by multiple enchondromas, generally in the region of the metaphysis and diaphysis (Fig. 18.19). If the skeleton is extensively affected, with predominantly unilateral distribution, the term Ollier disease is applied. The clinical manifestations of multiple enchondromas, such as knobby swellings of the digits or gross disparity in the length of the forearms or legs, are frequently recognized in childhood and adolescence; the disease has a strong preference for one side of the body. The disorder has no hereditary or familial tendency. Some investigators claim that it is not a neoplastic lesion but rather a developmental bone dysplasia. Maffucci syndrome is a congenital, nonhereditary disorder, characterized by enchondromatosis and soft-tissue angiomatosis (hemangiomatosis). The hemangiomas may occur anywhere in the skin and subcutaneous tissue. They are usually cavernous in type and may form unilaterally or bilaterally. The enchondromas in Maffucci syndrome have predilection for the tubular bones and have the same distribution as in Ollier disease, with a strong predisposition for one side of the body, the metacarpals and the phalanges being the most common sites. The pathogenesis of Ollier disease and Maffucci syndrome is unknown. The recent investigations, however, suggested that these disorders represent two entities within continuum of enchondromatosis and that both conditions bear the risk of mesodermal and nonmesodermal malignancy, caused by somatic mosaic mutations of IDH1 and IDH2 genes.
Conventional radiography is usually sufficient to demonstrate the typical features of enchondromatosis/Ollier disease. Characteristically, interference of the lesion with the growth plate causes foreshortening of the limbs. Deformity of the bones is marked by radiolucent masses
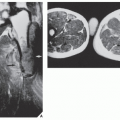
of cartilage, often in the hand and foot, containing foci of calcification (Fig. 18.20). Enchondromas in this location may be intracortical and periosteal. They sometimes protrude from the shaft of the short or long tubular bone, thus resembling osteochondromas (Fig. 18.21). Linear columns of cartilage in the form of radiolucent streaks extend from the growth plate to the diaphysis, and a fan-like pattern is common in the iliac bones (Fig. 18.22). MRI demonstrates lobulated in contour masses exhibiting low-to-intermediate signal intensity on T1-weighted images and high signal on T2 weighting. After injection of gadolinium, there is various degree of enhancement (Fig. 18.23). The Maffucci syndrome, in addition to the typical osseous alterations of enchondromatosis, is recognized radiographically by the presence of multiple calcified phleboliths (Fig. 18.24).
of cartilage, often in the hand and foot, containing foci of calcification (Fig. 18.20). Enchondromas in this location may be intracortical and periosteal. They sometimes protrude from the shaft of the short or long tubular bone, thus resembling osteochondromas (Fig. 18.21). Linear columns of cartilage in the form of radiolucent streaks extend from the growth plate to the diaphysis, and a fan-like pattern is common in the iliac bones (Fig. 18.22). MRI demonstrates lobulated in contour masses exhibiting low-to-intermediate signal intensity on T1-weighted images and high signal on T2 weighting. After injection of gadolinium, there is various degree of enhancement (Fig. 18.23). The Maffucci syndrome, in addition to the typical osseous alterations of enchondromatosis, is recognized radiographically by the presence of multiple calcified phleboliths (Fig. 18.24).
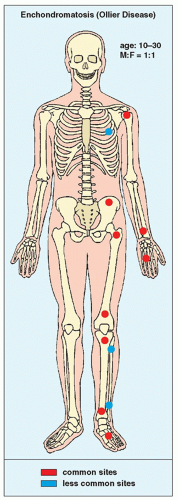 FIGURE 18.19 Skeletal sites of predilection, peak age range, and male-to-female ratio in enchondromatosis (Ollier disease). |
Histologically, the lesions of enchondromatosis are essentially indistinguishable from those of solitary enchondromas, although on occasion they tend to be more cellular.
Complications
The most frequent and severe complication of Ollier disease is malignant transformation to chondrosarcoma. In contrast to solitary enchondromas,
even lesions in the short tubular bones may undergo sarcomatous change (Fig. 18.25). This is also true in patients with Maffucci syndrome (Fig. 18.26).
even lesions in the short tubular bones may undergo sarcomatous change (Fig. 18.25). This is also true in patients with Maffucci syndrome (Fig. 18.26).
Osteochondroma
Also known as osteocartilaginous exostosis, this lesion is characterized by a cartilage-capped bony projection on the external surface of a bone. It is the most common benign bone lesion, constituting approximately 20% to 50% of all benign bone tumors, and is usually diagnosed in patients before their third decade. It has been postulated that sporadic osteochondromas represent developmental abnormality; however, recent cytogenetic studies revealed mutations in the EXT gene encoding exostosin 1, suggesting their neoplastic nature. Apparently, these genetic mutations lead to abnormal processing and accumulation of heparan sulphate proteoglycans (HSPG) in the cytoplasm of the chondrocytes. This leads to a loss of polar organization of the growth plate allowing chondrocytes to grow in the wrong direction. Continued growth of these chondrocytes coupled with endochondral ossification result in the formation of outpouching of both medullary and cortical bone covered by the cartilaginous cap, thus forming an exostosis. Osteochondroma, which has its own growth plate, usually stops growing at skeletal maturity. The most common sites of involvement are the metaphyses of the long bones, particularly in the region around the knee and the proximal humerus (Fig. 18.27). Variants of osteochondroma include subungual exostosis, turret exostosis, traction exostosis, bizarre parosteal osteochondromatous proliferation (BPOP), florid reactive periostitis, and dysplasia epiphysealis hemimelica (also called intraarticular osteochondroma or Trevor-Fairbank disease).
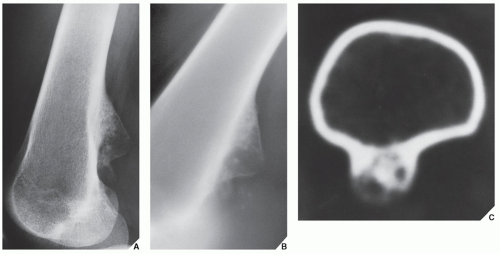
The radiographic presentation of osteochondroma is characteristic according to whether the lesion is pedunculated, with a slender pedicle usually directed away from the neighboring growth plate (Fig. 18.28A), or sessile, with a broad base attached to the cortex (Figs. 18.28B,C). The most important characteristic feature of either type of lesion is uninterrupted merging of the cortex of the host bone with the cortex of the osteochondroma; additionally, the medullary portion of the lesion and the medullary cavity of the adjacent bone communicate. CT scanning can establish unequivocally the lack of cortical interruption and the continuity of cancellous portions of the lesion and the host bone (Fig. 18.29). These are important features that distinguish this lesion from the occasionally similar looking bone masses of osteoma, periosteal chondroma, BPOP, juxtacortical osteosarcoma, soft-tissue osteosarcoma, and juxtacortical myositis ossificans (Fig. 18.30). The other characteristic feature of osteochondroma involves calcifications in the chondro-osseous portion of the stalk of the lesion (see Fig. 18.28) and cartilaginous cap. The thickness of the cartilaginous cap ranges from 1 to 3 mm and rarely exceeds 1 cm. On MRI, the cartilaginous cap shows high signal intensity on T2-weighted and gradientecho sequences. A narrow band of low signal intensity surrounding the cap represents the overlying perichondrium (Fig. 18.31).
Histologically, the osteochondroma cap is composed of hyaline cartilage arranged similarly to that of a growth plate. A zone of calcification in the chondro-osseous portion of the stalk corresponds to the zone of provisional calcification in the physis. Beneath this zone, there is vascular invasion and replacement of the calcified cartilage by new bone formation, which undergoes maturation and merges with the cancellous bone of the host bone’s medullary cavity.
Complications
Osteochondroma may be complicated by a number of secondary abnormalities, including pressure on nerves or blood vessels (Fig. 18.32), pressure on the adjacent bone (Fig.18.33; see also Fig. 16.66), with occasional fracture (Fig. 18.34), fracture through the lesion itself, and inflammatory changes of the bursa exostotica (“exostosis bursata”) covering the cartilaginous cap (Fig. 18.35).
The least common complication of osteochondroma, seen in solitary lesions in less than 1% of cases, is malignant transformation to chondrosarcoma.
Nevertheless, it is important to recognize this complication at an early stage. The chief clinical features suggesting malignant transformation are pain (in the absence of a fracture, bursitis, or pressure on nearby nerves) and a growth spurt or continued growth of the lesion beyond the age of skeletal maturity. Certain imaging features have also been identified that may help in the determination of malignancy (Table 18.1).
Nevertheless, it is important to recognize this complication at an early stage. The chief clinical features suggesting malignant transformation are pain (in the absence of a fracture, bursitis, or pressure on nearby nerves) and a growth spurt or continued growth of the lesion beyond the age of skeletal maturity. Certain imaging features have also been identified that may help in the determination of malignancy (Table 18.1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree