Benign Tumors and Tumor-like Lesions III: Fibrous, Fibroosseus, and Fibrohistiocytic Lesions
Fibrous Cortical Defect and Nonossifying Fibroma
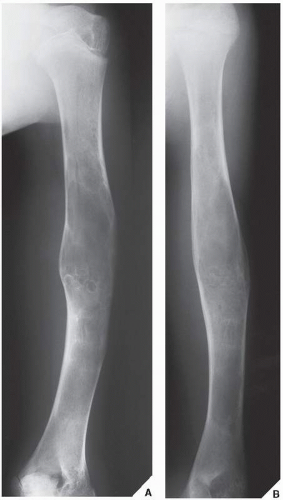
Fibrous cortical defects and nonossifying (nonosteogenic) fibromas are the most common fibrous lesions of bone and are predominantly seen in children and adolescents. More common in boys than in girls, they have a predilection for the long bones, particularly the femur and tibia (Fig. 19.1). Some authors prefer the term fibroxanthoma for both lesions, whereas Schajowicz prefers the term histiocytic xanthogranuloma. These lesions are not true neoplasms and are considered by many investigators developmental defects.
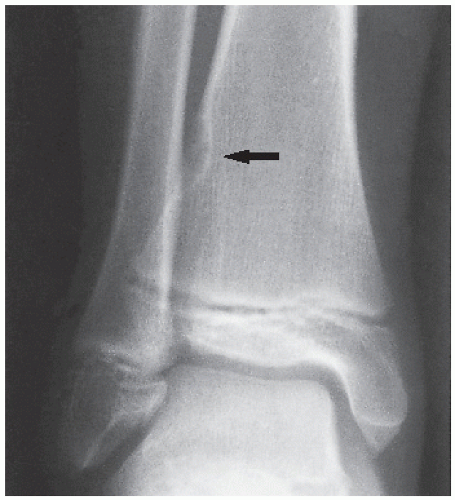
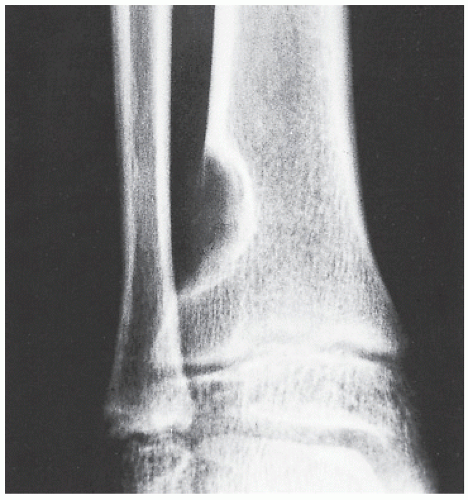
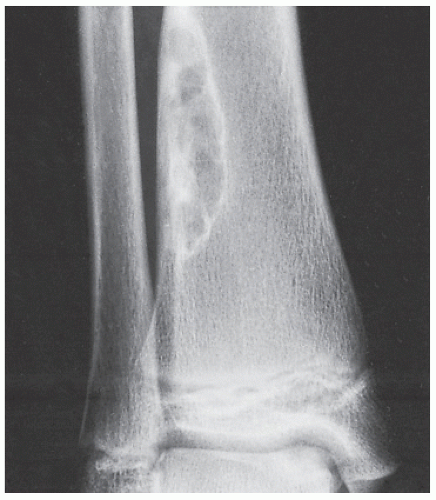
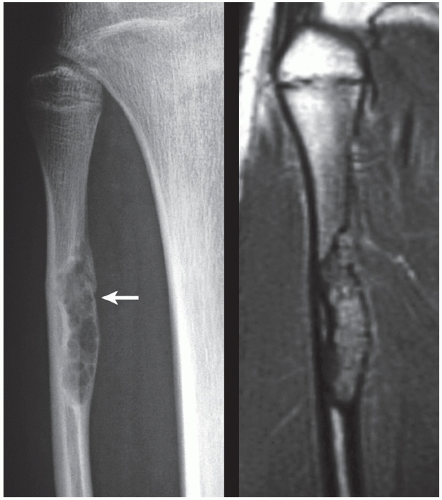
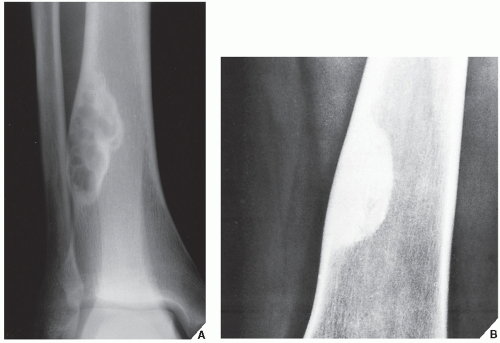
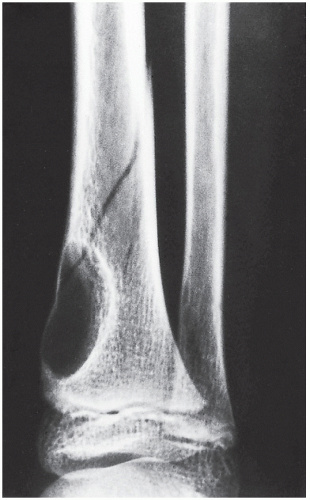
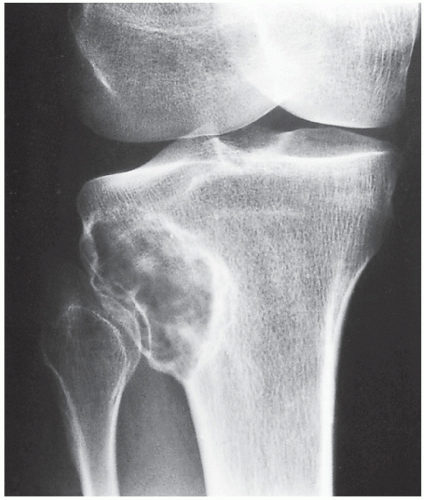
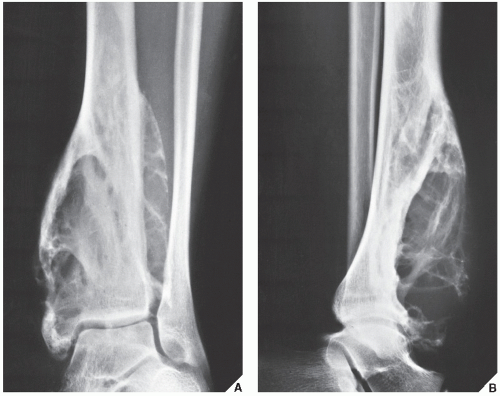
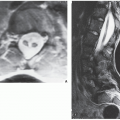
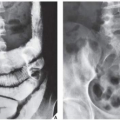
Fibrous cortical defect (metaphyseal fibrous defect) is a small asymptomatic lesion found in 30% of normal individuals in the first and second decades of life. The radiolucent lesion is elliptical and confined to the cortex of a long bone near the growth plate; it is demarcated by a thin margin of sclerosis (Figs. 19.2 and 19.3). Most of these lesions disappear spontaneously, but a few may continue to enlarge. When they encroach on the medullary region of a bone, they are designated nonossifying fibroma (Fig. 19.4). With continued growth, these lesions, which are typically located eccentrically in the bone, display a characteristic scalloped sclerotic border (Fig. 19.5).
Occasionally, nonossifying fibroma may involve several bones, in which case the condition is called disseminated nonossifying fibromatosis. Some of the patients with this presentation may exhibit on the skin café-au-lait spots with smooth (“coast of California”) borders, similar to those seen in neurofibromatosis. Furthermore, they may develop neurofibromas affecting varius nerves (see Chapter 33). This association is known as Jaffe-Campanacci syndrome (Fig. 19.6).
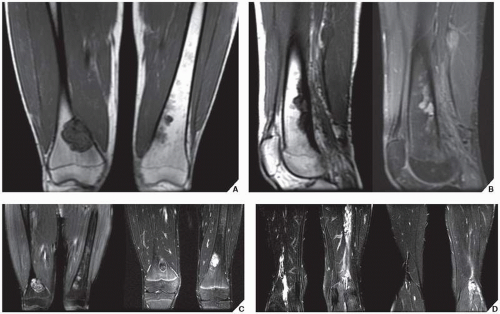
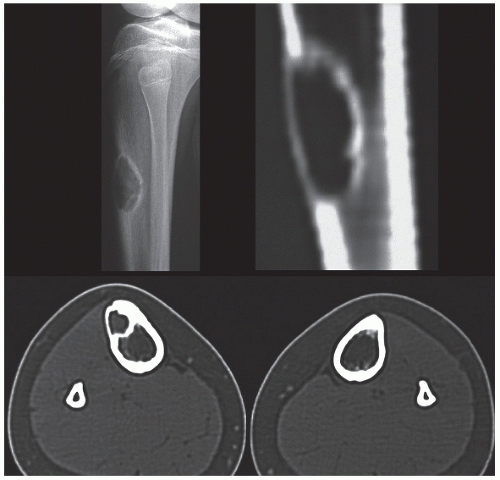
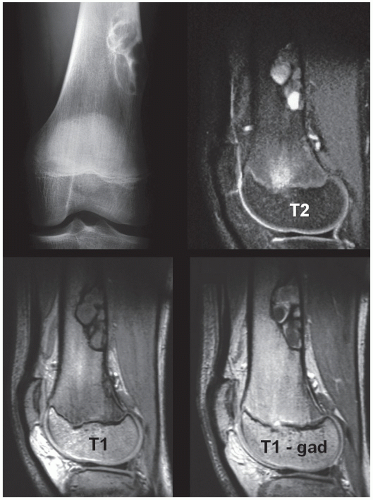
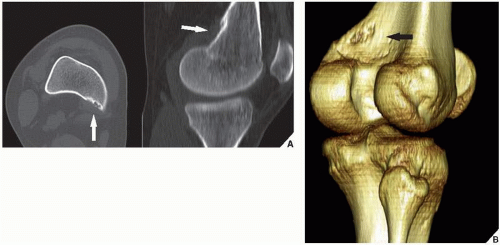
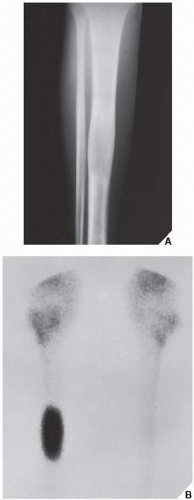
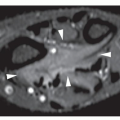
Skeletal scintigraphy shows a minimal to mild increase in activity. During the healing phase, mild hyperemia may be seen on the blood pool image, and the positive delayed scan reflects the osteoblastic activity. Computed tomography (CT) may demonstrate to better advantage the cortical thinning and medullary involvement (Fig. 19.7) and may delineate early pathological fracture more precisely. Hounsfield attenuation values for nonossifying fibroma are higher than for normal bone marrow. Magnetic resonance imaging (MRI), usually performed for another reason, shows intermediate to low signal intensity on T1-weighted and intermediate to high signal on T2-weighted sequences (Fig. 19.8). After gadolinium-diethylenetriamine-pentaacetic acid (DTPA) injection, both fibrous cortical defects and nonossifying fibromas invariably exhibit a hyperintense border and signal enhancement (Fig. 19.9). Mineralization of the lesion during healing appears predominantly as low signal intensity on MR images.
Histologically identical, regardless of size, fibrous cortical defect and nonossifying fibroma are composed of spindle and histiocytic cells that have a clear, foamy cytoplasm. In addition, osteoclast-like multinucleated giant cells are present, and varying numbers of inflammatory cells (lymphocytes) and plasma cells are scattered in the background. The cells are often arranged in a storiform pattern, typifying fibrohistiocytic lesions. Some lesions contain an excessive amount of fat within the foam cells, and the term xanthoma or fibroxanthoma may be applied to such lesions.
Complications and Treatment
Most lesions undergo spontaneous involution (healing) by sclerosis or remodeling (Fig. 19.10). Some larger lesions may be complicated by pathological fracture (Fig. 19.11). Therefore, if a lesion is large, extending across 50% or more of the medullary cavity, then curettage and bone grafting is the treatment of choice.
Benign Fibrous Histiocytoma
The term benign fibrous histiocytoma, although it may be controversial, is useful to subclassify lesions with histologic features similar to those of nonossifying fibroma but having an atypical clinical presentation and an atypical radiographic pattern. This lesion frequently has radiographic features very similar to those of nonossifying fibroma; it is radiolucent, with sharply defined and frequently sclerotic borders, without any mineralization of the matrix (Figs. 19.12 and 19.13). Its differentiation
from nonossifying fibroma is made on purely clinical grounds, because the histologic features of both lesions are almost identical. Patients presenting with benign fibrous histiocytoma are older (usually older than 25 years) than those with nonossifying fibroma; unlike the latter lesion, benign fibrous histiocytomas may produce symptoms such as pain or discomfort in the involved bone. These lesions also seem to run a more aggressive clinical course and may recur after treatment, which consists of curettage and bone grafting.
from nonossifying fibroma is made on purely clinical grounds, because the histologic features of both lesions are almost identical. Patients presenting with benign fibrous histiocytoma are older (usually older than 25 years) than those with nonossifying fibroma; unlike the latter lesion, benign fibrous histiocytomas may produce symptoms such as pain or discomfort in the involved bone. These lesions also seem to run a more aggressive clinical course and may recur after treatment, which consists of curettage and bone grafting.
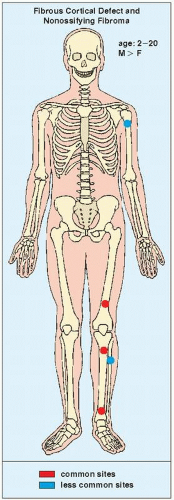 FIGURE 19.1 Skeletal sites of predilection, peak age range, and male-to-female ratio in fibrous cortical defect and nonossifying fibroma. |
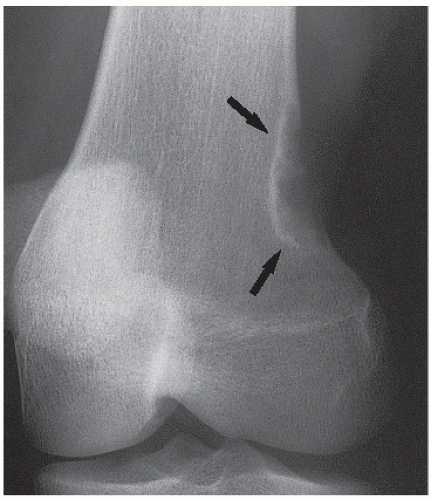 FIGURE 19.3 Fibrous cortical defect. A 21-year-old woman with a fibrous cortical defect affecting medial cortex of the distal femur (arrows). |
Periosteal Desmoid
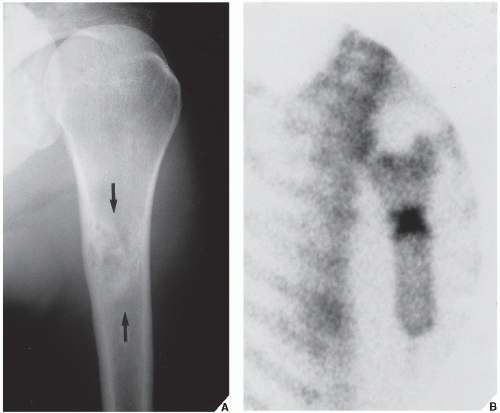
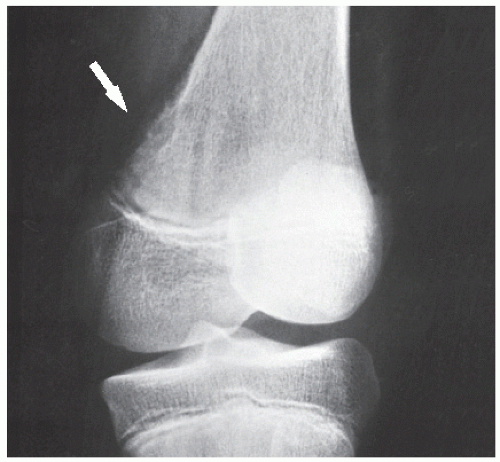
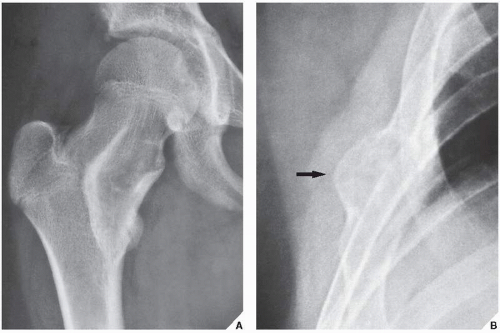
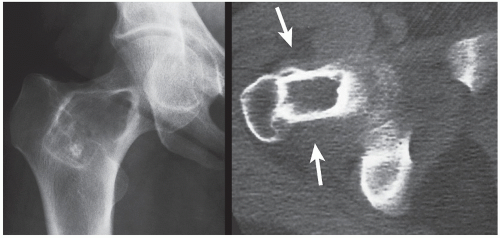
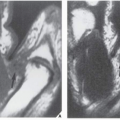
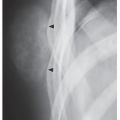
The periosteal desmoid is a tumor-like fibrous proliferation of the periosteum. It occurs in patients between the ages of 12 and 20 years and has a striking predilection for the posteromedial cortex of the medial femoral condyle. Many patients have a history of injury, although trauma is not necessarily a predisposing factor. The lesion simulates a fibrous cortical defect, except in the specificity of its location. Occasionally, it may simulate an aggressive and even malignant tumor. Radiographically, the hallmarks of a periosteal desmoid are its radiolucent saucer-shaped appearance, with sclerosis at the base eroding the cortex or producing cortical irregularity (Fig. 19.14). The radionuclide bone scan is usually normal, but sometimes may show a focal increase in activity. CT shows a well-defined lesion, commonly with a sclerotic border (Fig. 19.15). On MRI, the lesion appears hypointense on T1-weighted and hyperintense on T2-weighted images, with a dark rim on both sequences at or near the sites of the bony attachment of the medial head of the gastrocnemius muscle. Periosteal desmoid belongs to the “don’t touch” lesions (see Table 16.10), so it should not undergo biopsy. Most lesions disappear spontaneously by the time the patient reaches age 20.
The histologic appearance of the lesion demonstrates fibroblastic spindle cells that produce large amounts of collagen. Large areas of hyalinization and fibrocartilage and small fragments of bone may be scattered within the fibrous tissue.
Differential Diagnosis
Some authorities believe that periosteal desmoid should be differentiated from distal femoral cortical irregularity. This latter abnormality, which presents as cortical roughening just distal to the extension of the linea aspera, is a common finding in boys in the 10- to 15-year age group. Its cause is not settled. Although it was thought to represent an avulsion injury caused by traction of the adductor magnus aponeurosis, Brower and colleagues have shown that this lesion may exist in the area without any muscular or ligamentous attachment. Others consider periosteal desmoid and distal femoral cortical irregularity to be the same entity. Dahlin suggests that the periosteal desmoid is a hypocellular variant of nonossifying fibroma, and Schajowicz classifies it as a periosteal variant of desmoplastic fibroma. Other authors apply a broader definition to periosteal desmoid, considering it essentially a hypocellular variant of fibrous cortical defect. In any event, it is a self-limited benign lesion that requires no treatment, and its characteristic imaging appearance and location should serve as clues to the correct diagnosis.
Fibrous Dysplasia
Fibrous dysplasia, occasionally termed fibrous osteodystrophy, osteodystrophia fibrosa, or osteitis fibrosa disseminata, is a fibroosseous lesion that some authorities classify among the group of developmental dysplasias. At present time, however, it is considered to be a genetically based sporadic disorder due to mutation in the GNAS1 gene, the defect that prevents osteoblasts to form a normal lamellar bone. There are two common GNAS1 mutations associated with fibrous dysplasia, both occuring at codon 201, with argenine being substituted for either cysteine or histidine, R201C and R201H respectively. Most recently reported the third GNAS1 gene mutation (Q227L) represent only about 5% of the GNAS1 mutation in this condition. It may affect one bone (monostotic form) or several bones (polyostotic form). Fibrous dysplasia is characterized by the replacement of normal lamellar cancellous bone by an abnormal fibrous tissue that contains small, abnormally arranged trabeculae of immature woven bone formed by metaplasia of the fibrous stroma.
Monostotic Fibrous Dysplasia
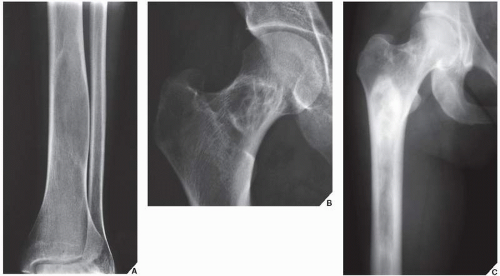
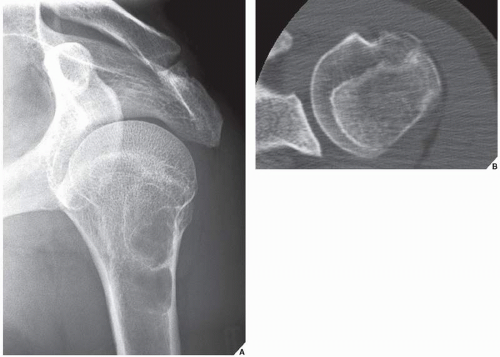
Monostotic fibrous dysplasia most commonly affects the femur—particularly the femoral neck—as well as the tibia and ribs (Fig. 19.16). The lesion arises centrally in the bone, usually sparing the epiphysis in children, and it is very rarely seen in the articular end of the bone in adults (Fig. 19.17). As the lesion enlarges, it expands the medullary cavity. The radiographic appearance of monostotic fibrous dysplasia varies, depending on the proportion of osseous-to-fibrous content. Lesions with greater osseous content are more dense and sclerotic, whereas those with greater fibrous content are more radiolucent, with a characteristic ground-glass appearance (Figs. 19.18 and 19.19, see also Fig. 19.16B). One of the lesions that mimics monostotic fibrous dysplasia, particularly when located in the intertrochanteric region of the femur, is the so-called liposclerosing myxofibrous tumor (Fig. 19.20), a benign fibroosseous lesion characterized by a complex mixture of histologic elements that include lipoma, fibroxanthoma, myxoma, myxofibroma, fat necrosis, bone, and cartilage.
Scintigraphy is helpful in determining the activity of fibrous dysplasia (Fig. 19.21) and the potential multicentricity of the lesion. Machida and associates reported that although a high incidence to show similarly increased uptake.
The CT findings parallel those of conventional radiography. CT sections show areas of high attenuation in more sclerotic lesions and a low-attenuation matrix with an amorphous ground-glass texture in lesions with greater fibrous content (Figs. 19.22 and 19.23). The lesion of fibrous dysplasia shows a variety of appearances on MRI caused by the histologic composition of these lesions. Some lesions show a decreased signal on T1 and T2 sequences, and some show intermediate or low signal on T1-weighted but either mixed or high signal on T2-weighted images. The sclerotic rim (rind sign) is invariably imaged as a band of low signal intensity on T1 and T2 sequences.
Pathological fracture of the structurally weakened bone is the most frequent complication of monostotic fibrous dysplasia.
Histologically, fibrous dysplasia presents as an aggregate of moderately dense fibrous connective tissue containing bony trabeculae in haphazard distribution instead of the stress-oriented distribution expected in normal cancellous bone. The trabeculae are curved and branching, with sparse interconnections. Low-power photomicrographs have been likened to “alphabet soup” or Chinese ideographs. They are composed of woven, immature bone and exhibit no evidence of osteoblastic activity (“naked trabeculae”). Occasionally, an area of cartilage formation may be present within the lesion.
Polyostotic Fibrous Dysplasia
Although radiographically similar to the monostotic form, polyostotic fibrous dysplasia is a more aggressive disorder. It also has a different distribution in the skeleton and a striking predilection for one side of the body (Fig. 19.24), a tendency that has been noted in more than 90% of cases. The pelvis is frequently affected, followed by the long bones, skull, and ribs; the proximal end of the femur is a common site of involvement (Fig. 19.25). The lesions generally
progress in number and size until the end of skeletal maturation, at which time they become quiescent. In only 5% of cases do they continue to enlarge.
progress in number and size until the end of skeletal maturation, at which time they become quiescent. In only 5% of cases do they continue to enlarge.
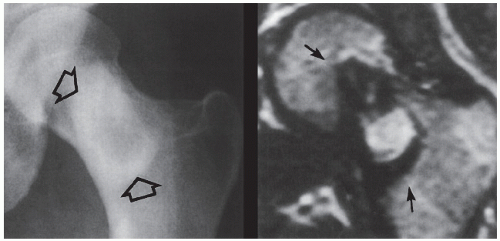 FIGURE 19.20 Liposclerosing myxoid tumor. Anteroposterior radiograph of the left hip of a 38-year-old woman presenting with vague hip pain shows a radiolucent lesion with well-defined thick scleroting border in the intertrochanteric region of the femur (open arrows). Coronal T2-weighted MR image shows the lesion (arrows) to exhibit heterogeneous signal intensity. Peripheral sclerotic “rind” displays signal void. (Reprinted with permission from Kransdorf MJ et al., 1999.) |
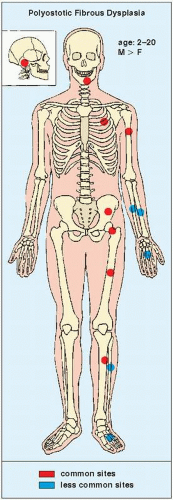 FIGURE 19.24 Skeletal sites of predilection, peak age range, and male-to-female ratio in polyostotic fibrous dysplasia, which is usually seen in only one side of the skeleton. |
Radiographically, the changes typical of fibrous dysplasia may occur in a limited segment or a major portion of the long bones affected by the polyostotic form of the disease, but as in the monostotic form, the articular ends are usually spared. The cortex, which is generally left intact, is often thinned by the expansive component of the lesion, and the inner cortical margins may show scalloping. The lesion has a well-defined border. Occasionally, as in the monostotic form, the replacement of medullary bone by fibrous tissue leads to a loss of the trabecular pattern, giving the lesions a ground-glass, “milky,” or “smoky” appearance (see Fig. 19.18A). More osseous lesions appear dense. The quickest means of determining the distribution of the lesion in the skeleton is radionuclide bone scan, which often discloses unsuspected sites of skeletal involvement (Fig. 19.26).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree